Signalment:
Gross Description:
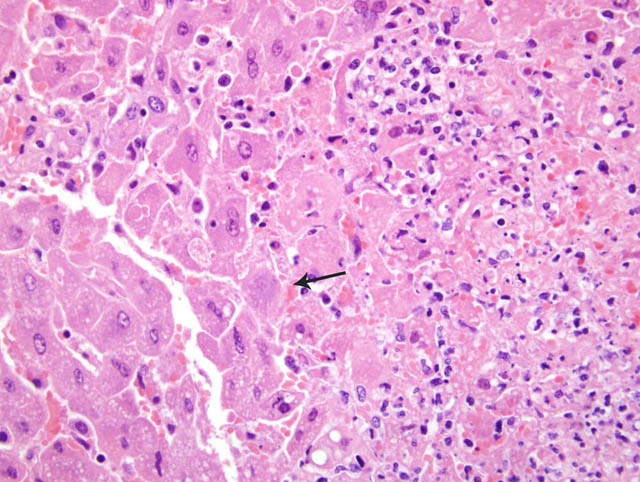
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Tyzzers disease is common in laboratory animals including mice, rats, guinea pigs, and rabbits. C. piliforme is considered a commensal organism in the rodent intestinal tract which may spread to the liver via the portal circulation.5 In laboratory mice systemic infection is often subclinical with fulminate disease occurring sporadically. Susceptibility varies among mouse strains. Likewise, virulence varies among C. piliforme isolates. Factors involved in the immunity and virulence have been investigated in mice.16,17,18 Infection of both susceptible and resistant strains of mice with either toxigenic (virulent) or non-toxigenic C. piliforme isolates resulted in strain-independent and isolate-independent elevations in serum IFN _ and IL-6 from day 1 to 14 and in serum TNF _ from day 1 to 28.16, 17 All mice had serologic evidence of inflammation; however, only mice infected with the toxigenic bacterial isolate had histologic lesions in the liver (day 7 to 14). A similar experiment revealed that IL-12 levels were significantly higher in resistant mice than susceptible mice.18 The importance of IL-6 and IL-12 in mediating the immune response to C. piliforme was also demonstrated in these experiments; histopathologic lesions in the liver were more severe if polyclonal antibodies against these cytokines were injected immediately prior to infection with the bacteria.17,18
The possibility of latent infections has been debated. Presence of bacterial DNA in hepatocytes from animals with elevated serum cytokines and normal liver histology suggests that the infection may persist in a latent state for long periods of time (at least 28 days).16
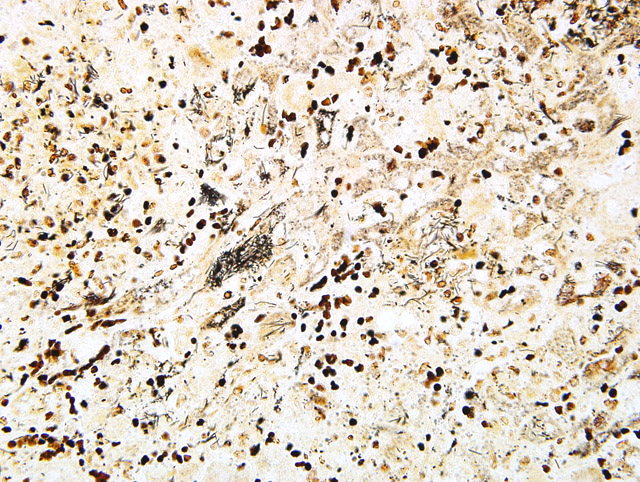
Special stains to identify the organisms are occasionally required. Warthin-Starry, Giemsa, Gomori Methamine Silver (GMS), and gram stains are commonly used for these purposes.5 In our laboratory, Warthin-Starry demonstrated the organisms (Fig. 1-2) as well as occasional short rods. This second infectious organism may represent a secondary bacterial infection, possibly one that ascended from the gastrointestinal tract.
JPC Diagnosis:
Conference Comment:
Transmission of Tyzzers disease is presumed to occur through ingestion of contaminated feces, with colonization of the intestine followed by hematogenous spread to the liver via the portal circulation.4 The mechanism of attachment and entry into the host cell is not currently known.4,5 C. piliforme is considered difficult to grow on artificial media and requires eggs or cell culture to proliferate.5,14 Diagnosis of infection is dependent on identification of the organism within the cytoplasm of degenerate and apparently healthy cells at the periphery of areas of necrosis. The bacteria have often been described as being arranged in characteristic sheaves, haystacks, or bundles. Immunohistochemistry, immunofluorescence, and PCR have all been used to aid in identification.14
Susceptibility to infection and disease progression has been linked to genetics, immune status, age, and bacterial virulence factors. T lymphocyte, natural killer cell, neutrophil cell function and cytokine responsiveness have all been linked to disease progression.10,18 The pathogenesis of C. piliforme infection does not appear to be clearly dependent on toxin production. Some strains do produce cytotoxic proteins and these isolates are generally more virulent than the non-toxic isolates.2,16
References:
2. Brown CC, Baker DC, Barker IK: Alimentary system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, p. 214. Elsevier Limited, St. Louis, MO, 2007
3. Canfield PJ, Hartley WJ: Tyzzers disease (Bacillus piliformis) in Australian marsupials. J Comp Pathol 105:167-173, 1991
4. Cullen JM: Liver, biliary system and exocrine pancreas. In: Pathologic Basis of Veterinary Disease, eds McGavin MD, Zachary JF, 4th ed., pp 433-434. Mosby Elsevier, St. Louis, Missouri, 2007
5. Jones BR, Greene CE: Tyzzers disease. In: Infectious Diseases of the Dog and Cat, ed. Greene CE, 3rd ed., pp. 362-363. Saunders Elseiver, St. Louis, Missouri, 2006
6. Langan J, Bemis D, Harbo S, Pollock C, Schumacher J: Tyzzers disease in a red panda (Ailurus fulgens fulgens). J Zoo Wildl Med 31:558-562, 2000
7. Marler RJ, Cook JE: Tyzzers disease in two coyotes. J Am Vet Med Assoc. 169:940-941, 1976
8. Poonacha KB: Naturally occurring Tyzzers disease in a serval (Felis capensis). J Vet Diagn Invest 9:82-84, 1997
9. Raymond JT, Topham K, Shirota K, Ikeda T, Garner MM: Tyzzers disease in a neonatal rainbow lorikeet (Trichoglossus haematodus). Vet Pathol 38:326-327, 2001
10. Sasseville VG, Simon MA, Chalifous LV, Lin KC, Mansfield KG: Naturally occurring Tyzzers disease in cotton-top tamarins (Sanguinus oedipus). Comp Med 57:125-127, 2007
11. Saunders GK, Sponenberg DP, Marx KL: Tyzzers disease in a neonatal cockatiel. Avian Dis 37:891-894, 1993
12. Schmidt RE, Eisenbrandt DL, Hubbard GB: Tyzzers disease in snow leopards. J Comp Pathol 94:165-167, 1984
13. Smith KJ, Skelton HG, Hilyard EJ, Hadfield T, Moeller RS, Tuur S, Decker C, Wagner KF, Angritt P: Bacillus piliformis infection (Tyzzers disease) in a patient infected with HIV-1: confirmation with 16S ribosomal RNA sequence analysis. J Am Acad Dermatol 34:343-348, 1996
14. Stalker MJ, Hayes MA: Liver and biliary system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, p. 356. Elsevier Limited, St. Louis, MO, 2007 15. Stanley SM, Flatt RE, Daniels GN: Naturally occurring Tyzzers disease in the gray fox. J Am Vet Med Assoc 173:1173-1174, 1978
16. Van Andel RA, Franklin CL, Besch-Williford, CL, Hook RR, Riley LK: Prolonged perturbations of tumor necrosis factor- _ and interferon-_ in mice inoculated with Clostridium piliforme. J Med Microbiol 49:557-563, 2000
17. Van Andel RA, Franklin CL, Besch-Williford, CL, Hook RR, Riley LK: Role of interleukin-6 in determining the course of murine Tyzzers disease. J Med Microbiol 49:171-176, 2000
18. Van Andel RA, Hook RR, Franklin CL, Besch-Williford, CL, Riley LK: Interleukin-12 has a role in mediating resistance of murine strains to Tyzzers disease. Infection and Immunity 66:4943-4946, 1998
19. Wobeser G, Barnes HJ, Pierce K: Tyzzers disease in muskrats: re-examination of specimens of hemorrhagic disease collected by Paul Errington. J Wildl Dis 15:525-527, 1979
20. Wojcinski ZW, Barker IK: Tyzzers disease as a complication of canine distemper in a raccoon. J Wildl Dis 22:55-59, 1986