Signalment:
Adult, intact female, great blue heron (
Ardea herodias)The heron was presented to the wildlife clinic with a poor body condition score (1.5/5), as well as being dehydrated and weak. The heron was initially treated with metacam, claforan, and lactated Ringers solution. Over the next 24 hours the heron developed subcutaneous edema ventral to the bill and was started on colloid therapy (Hetastarch). The heron failed to improve and was found dead 36 hours after presentation.
Gross Description:
The pectoral muscles were moderately atrophied and the keel was prominent on palpation. The subcutaneous tissues ventral to the bill down to the thoracic inlet were moderately edematous. The ventral serosal surface of the proventriculus and adjacent ventriculus was focally overlain by a mass of multiple, tortuous intertwining tubules and a few ovoid cysts. Tubule diameters were up to 2 mm and cysts measured up to 1 cm long X 0.5 cm in diameter. The tubules and cysts were filled with dark red to brown, crumbly, soft debris and multiple pale nematodes 0.5 mm in diameter and up to 3 cm long. The ventriculus contained scant digested blood.
Histopathologic Description:
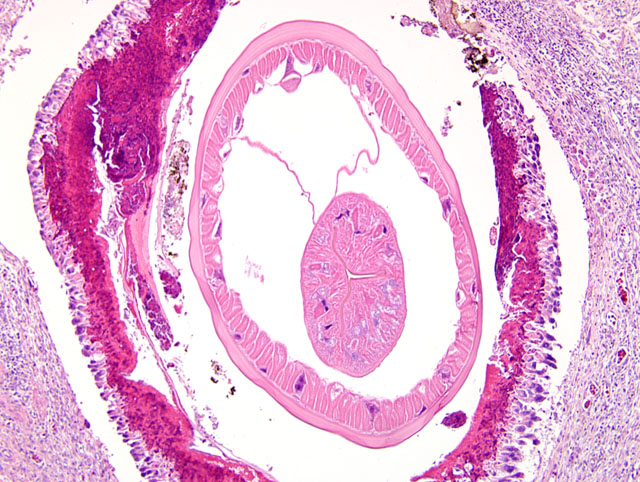
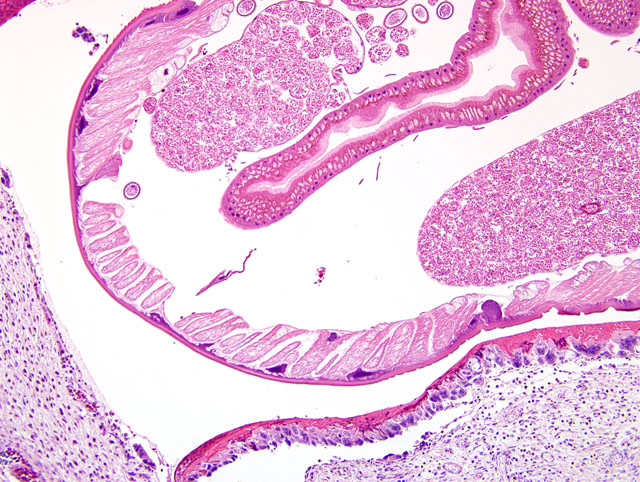
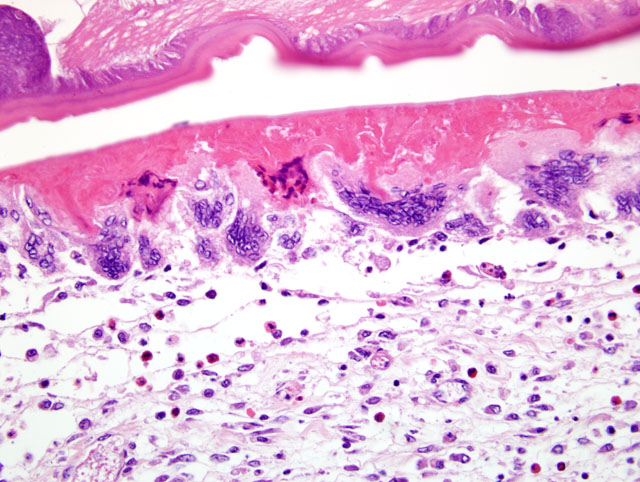
The wall of the proventriculus, including the mucosa, muscularis, and serosa and the adjacent mesentery contain multiple variably sized granulomas. The core of each granuloma consists of a layer of eosinophilic, granular and necrotic debris mixed with degenerate eosinophils and heterophils surrounding longitudinal and cross sections of well to poorly preserved nematodes. The layer of eosinophilic debris is, in turn, surrounded by a prominent layer of macrophages and multinucleated giant cells. The outermost layer is a thick but sparsely collagenous, reticular capsule composed of both immature and mature fibrocytes. Scattered throughout the capsule are eosinophils and macrophages, fewer lymphocytes, plasma cells, and heterophils, and small foci of hemorrhage. The capsule is multifocally intersected by variably sized vessels lined by plump endothelial cells. Structural characteristics of the nematodes include one or more of the following: a thick, eosinophilic, smooth cuticle; polymyarian-coelomyarian musculature; a pseudocoelom; a ventral nerve cord; a glandular esophagus with associated pseudomembranes; many thick shelled, operculated, oval eggs 70 microns X 40 microns; an intestinal tract lined by tall columnar cells; and male or female reproductive tracts (
Figs. 2-1, 2-2). Occasionally, the profile of nematodes is obscured and replaced by eosinophilic necrotic debris, degenerate granulocytes, and/or colonies of bacilli along with free floating nematode eggs. The proventriculus contains multiple aggregates of eosinophils, macrophages, and lymphocytes and is mildly edematous. Glands of the proventriculus often contain sloughed epithelial cells, granular eosinophilic debris, and few leukocytes. The mesentery is mildly edematous and adipose tissue is atrophied.
Morphologic Diagnosis:
Proventriculus: Proventriculitis, chronic-active, granulomatous (
Fig. 2-3), eosinophilic, and heterophilic, multifocal, moderate, with multifocal mesenteric granulomas, serous atrophy of fat and intralesional adult nematodes consistent with
Eustrongylides sp.
Lab Results:
Initial laboratory results:
Blood glucose = 297
PCV = 45
Total protein = 3.8
Fecal floatation detected ascarids
Condition:
Eustrongylides ignotus
Contributor Comment:
Eustrongylides sp. infections have been reported from birds throughout the world and have been implicated as a significant cause of morbidity and mortality in wading birds.(1,4,5,7,9,11)
Eustrongylides undergo four developmental stages and require two intermediate hosts. Ciconiiformes (wading birds) are a definitive host for
Eustrongylides (11) and shed eggs in the feces into the environment. The first larval stage then develops within the eggs and is consumed by freshwater oligochaetes (worms). The eggs then hatch and develop into second and third stage larvae. Small fish feed on the oligochaetes and the third stage larvae become encysted within the fish and develop into the infective fourth stage larvae. Wading birds then consume the fish and become infected. Alternatively, transport hosts, including amphibians, reptiles, and other fish, consume the smaller fish with the fourth stage larvae and then are consumed by wading birds. Larvae penetrate the wall of the proventriculus and ventriculus within 3 to 5 hours following ingestion of infected fish (7) and become adults in 2 to 8 days.(7) The shedding of eggs has been reported 10-17 days (1) and 14-23 days (7) post infection.Â
Eustrongylides have no oral structures to allow attachment to the mucosal surface of the gastrointestinal tract, which may explain the rapid penetration of the proventriculus and ventriculus as well as clinical signs of regurgitation and vomiting as a way to remove the parasite.(7) Additional clinical signs reported include lethargy, depression, and emaciation.(11)
Significant lesions are frequently observed in birds infected with
Eustrongylides.(4) Gross lesions reported following acute infections include minimal inflammation and hemorrhage, perforations of the proventriculus, ventriculus, and other organs, hematoma formations, severe fibrinous coelomitis involving multiple visceral organs, necrosis, and/or the presence of nematodes in the coelom.(2,4,5,7,8,11) Histologic evaluation of tissues show a severe granulomatous response including heterophils, macrophages, and multinucleated giant cells surrounding the organisms, hemorrhage or hematomas, fibrinous coelomitis with or without bacteria, and necrosis.(1,4,7,11) Lesions associated with more chronic infections include firm granulomas, air sacculitis, large, intertwining, firm, pale tan tubules crossing over the serosal surfaces of multiple organs often containing intact or decomposing organisms and/or necrotic debris.(1,4,7,11) Tubules are usually patent and open into the ventriculus.(7) Histologic lesions include the presence of intact and degenerate nematodes surrounded by a zone of cellular debris, bacteria, fragments of cuticle, and eggs which, in turn, is surrounded by eosinophilic debris, multinucleated giant cells, and fibrous connective tissue.(7) Active inflammation may also be present on the surface of the tubules.(6)
Eustrongylidosis in ciconiiformes is caused by three species of
Eustrongylides: E. tubifex, E. ignotus, and
E. excisus, and the prevalence of
Eustrongylides sp. varies worldwide. A survey of ciconiiformes in Brazil revealed a 31% infection rate.(4) In the United States, eustrongylidosis has been implicated as a cause or contributor to losses of colonies of wading birds in Indiana (9), Delaware (8, 11), Louisiana (5), Florida (6), Texas (1), and Virginia.(1) In one survey from Florida, 13% of nestling wading birds and 24% of adult wading birds were infected.(6) The most commonly infected birds in that survey were adult Great blue herons (51%) followed by adult and nestling Great egrets (34% and 21%, respectively). 35% of nestlings in Texas, 6% in California, and 4% in Rhode Island were infected in a separate survey.(2) High mortality due to
Eustrongylides ignotus has been observed in Delaware, where the mortality rate reached 84% in snowy egret nestlings.(8) Multiple surveys have shown that nestlings and hatchlings are particularly sensitive and have high rates of mortality.(1,6,8,11) Infected adult birds, on the other hand, have lower rate of mortality, which serves as a source of continued shedding of eggs into the environment.(6) This can result in the reinfection of ciconiiformes, significantly impacting the overall health and reproductive success of wading bird colonies. Furthermore, the report of a human infection by
Eustrongylides sp. resulting from the consumption of sushi (10) highlights the zoonotic potential of this parasitic nematode.
JPC Diagnosis:
Proventriculus: Proventriculitis, granulomatous, multifocal, moderate with nematodes consistent with
Eustrongylides sp.
Conference Comment:
Eustrongylides species are nematodes belonging to the subclass Aphasmidia, which have very characteristic morphologic features. These parasites have stichosomes, which are basophilic structures that surround the esophagus. The stichosome is formed from a row of cells called stichocytes, which are esophageal gland cells.(3) Aphasmids also have a bacillary band, which is a boomerang shaped, densely basophilic band of nuclei present in the hypodermis. Adult female aphasmids have only one genital tract in contrast to two tracts in phasmid nematodes. Eggs in most aphasmid species have bipolar plugs. Commonly encountered aphasmid parasites include
Trichuris sp.,
Eustrongylides sp.,
Dioctyophyma sp., and
Capillaria sp.
References:
1. Cole RA: Eustrongylidosis.Â
In: Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds, Biological Resources Division, ed. Ciganovich EA, pp. 223-228: United States Geological Survey, United States Department of the Interior, Washington, DC, 1999
2. Franson JC, Custer TW: Prevalence of eustrongylidosis in wading birds from colonies in California, Texas, and Rhode Island, USA. Colonial Waterbirds
17:168-172, 1994
3. Gardiner CH, Poynton SL:
In: An Atlas of Metazoan Parasites in Animal Tissues, 2nd ed., pp. 40. Armed Forces Institute of Pathology, Washington, DC, 1998
4. Pinto RM, Barros LA, Tortelly L, Teixeira RF, Gomes DC: Prevalence and pathology of helminths of ciconiiform birds from the Brazilian swamplands. Journal of Helminthology
78:259-64, 2004
5. Roffe TJ: Eustrongylides sp. epizootic in young common egrets (
Casmerodius albus). Avian Diseases 32:143-7, 1988
6. Spalding MG, Bancroft GT, Forrester DJ: The epizootiology of eustrongylidosis in wading birds (ciconiiformes) in Florida. Journal of Wildlife Diseases
29:237-49, 1993
7. Spalding MG, Forrester DJ. Pathogenesis of eustrongylides ignotus (nematoda: Dioctophymatoidea) in ciconiiformes. Journal of Wildlife Diseases
29:250-60, 1993
8. Wiese JH, Davidson WR, Nettles VF: Large scale mortality of nestling ardeids caused by nematode infection. Journal of Wildlife Diseases
13:376-382, 1977
9. Winterfield RW, Kazacos KR: Morbidity and mortality of great blue herons in Indiana caused by eustrongylides ignotus. Avian Diseases
2:448-51, 1977
10. Wittner M, Turner JW, Jacquette G, Ash LR, Salgo MP, Tanowitz HB: Eustrongylidiasis-a parasitic infection acquired by eating sushi. The New England Journal of Medicine
320:1124-1126, 1989
11. Ziegler AF, Welte SC, Miller EA, Nolan TJ: Eustrongylidiasis in eastern great blue herons (
Ardea herodias). Avian Diseases
44:443-8, 2000