CASE 3: 18N2812 (4135943-00)
Signalment:
Adult, 20+ year-old Quarter Horse gelding, Equus caballus
History:
A 20+ year old Quarter horse gelding presented for a fever of unknown origin, anorexia and approximately 1-week history of nasal discharge and cough following multiple horses on the property confirmed to have influenza. Non-steroidal ant-inflammatories (NSAIDs) were not given prior to presentation. On abdominal ultrasound there was an abnormal echotexture and shape to the right kidney. Blood creatinine levels were also elevated (5.3 mg/dl; reference range: 0.9-2.0 mg/dl). Creatinine levels continued to rise in the face of fluid therapy. Due to poor prognosis, the owners elected for humane euthanasia.
Gross Pathology:
The pleural cavity contains approximately 1 liter of serosanguinous effusion. The lungs are mottled pale to dark pink. Approximately 25% of the cranioventral lung lobes are bilaterally firm and consolidated and sinks in formalin. The caudodorsal lung lobes are bilaterally soft to rubbery and floats in formalin. On cut section, the lung oozes clear, watery fluid.
There is approximately 1 liter of yellow to light brown, translucent, watery fluid present in the peritoneal cavity. Beneath the renal capsule, randomly, scattered over the entire surface of kidneys are hundreds of petechiae which range from 1 to 2 mm in diameter. On cut section the entire cortex of each kidney is similarly affected. Diffusely, the corticomedullary junctions are dark red. The renal pelvices, bilaterally, contains, pale tan, folds of undulating tissue, which is expanded by clear, watery to gelatinous, fluid. Segmentally, 6.5 cm of the right proximal ureter and 5.0 cm of left proximal ureter are transmurally red. The serosal and mucosal vasculature is prominent and congested at the apex of the bladder. Additionally, within this affected region of bladder mucosa there is a 0.5 cm in diameter purpura. The lumen of the bladder contains cloudy, dark yellow to brown urine.
Laboratory results:
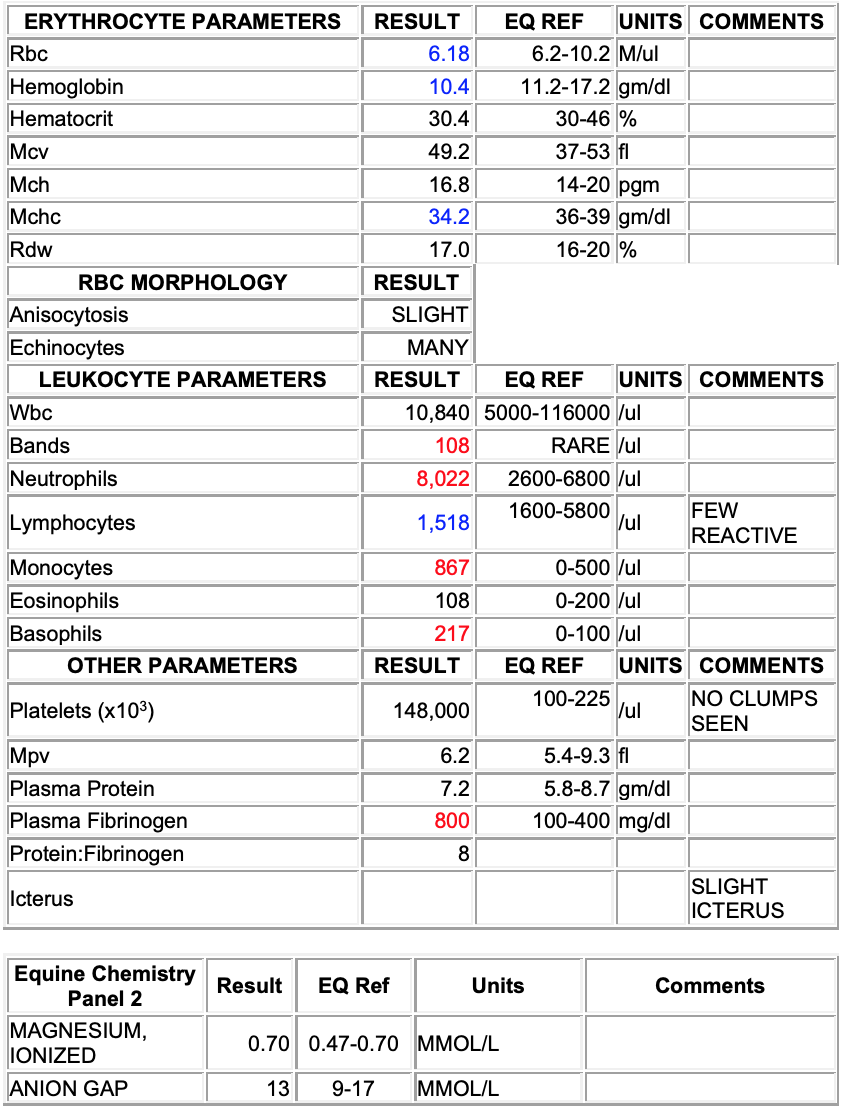
CBC:
Echinocytosis (many)
Left shift (bands; 108/ul)
Mild monocytosis (867/ul)
Mild basophilia (217/ul)
Fibrinogenemia (800 mg/dl)
Interpretation: Inflammatory leukogram
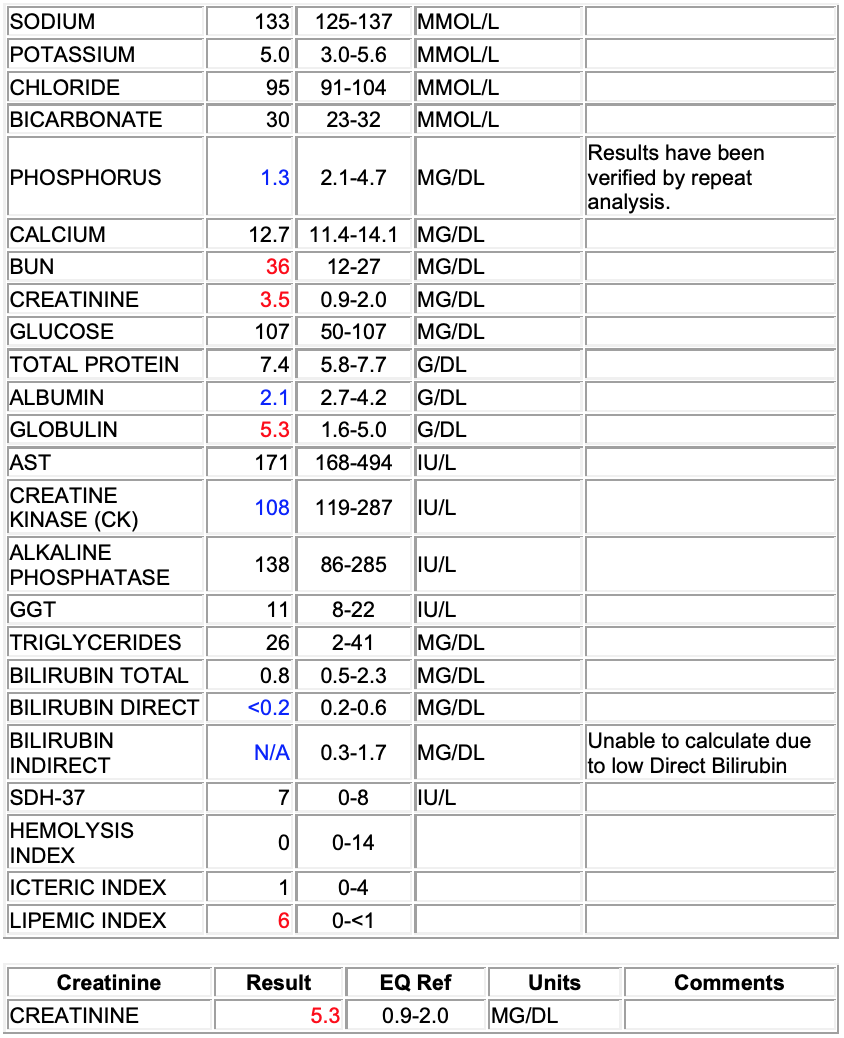
Chem:
Hypophosphatemia (1.3 mg/dl)
Azotemia: elevated BUN (36 mg/dl) and creatinine (5.3)
Hypoalbuminemia (2.1 g/dl)
Hyperglobulinemia (5.3 g/dl)
Low creatinine kinase (108 IU/L)
Hypobilirubinemia (<0.2 mg/dl direct)
Interpretation: Hypoalbuminemia, considerations include loss (renal or GI) or decreased production (no support). Histopathology suggests glomerular disease and therefore renal loss. Azotemia may be pre-renal, but histopathology suggest renal is primary. Hypophosphatemia may be secondary to alkalosis, supported by respiratory disease observed on necropsy, but renal losses and GI malabsorption cannot be fully excluded. Recommend blood gas, urinalysis and fractional excretion of phosphorous.
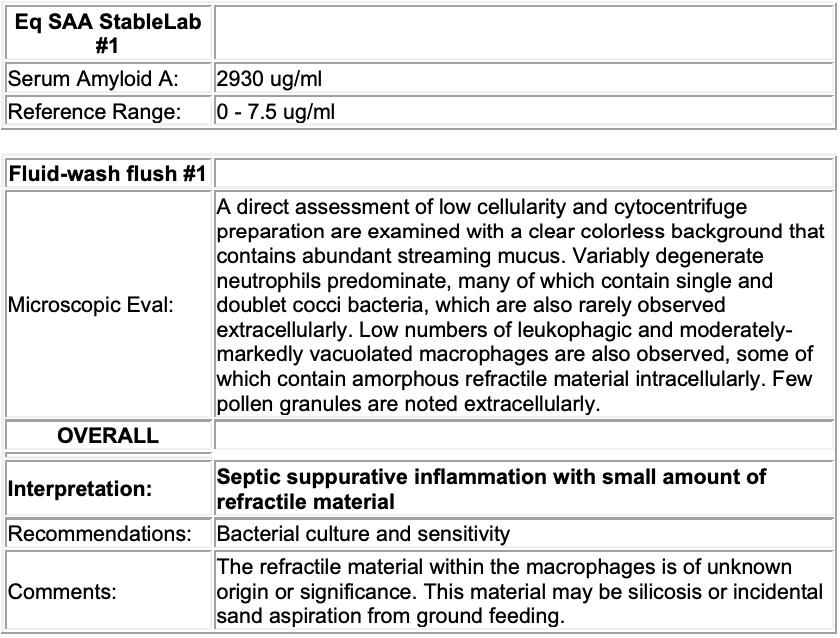
SAA:
Elevated serum amyloid A (SAA); (2930 ug/ml)
Interpretation: Inflammation
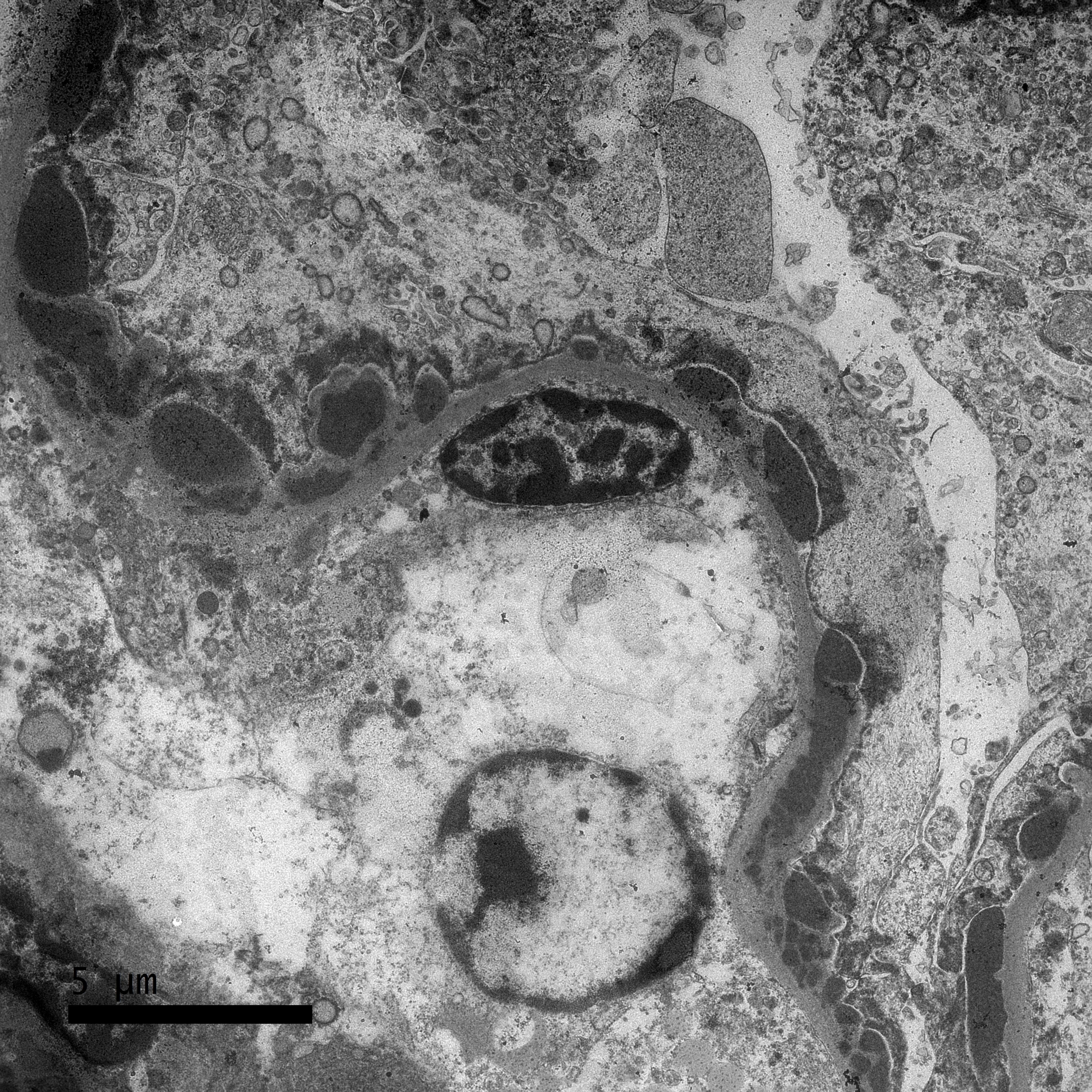
Transmission Electron Microscopy
A sample of the renal cortex had been submitted for electron microscopy. Three glomeruli are evaluated. Throughout the glomerulus are large numbers of subepithelial, intramembranous, subendothelial, and perimesangial dense (immune) deposits. Mesangial cell interpositioning underneath the capillary loop basement membrane is observed in one location. Podocyte cell bodies are swollen and have foot process effacement. Rare lipid vacuoles are present in tubular epithelial cells.
Microbiology
Lung: A sample of the right cranial lung was submitted for aerobic bacterial culture and grew moderate numbers of Streptococcus equi subsp. zooepidemicus and very small numbers of Staphylococcus aureus.
Kidneys: Samples of kidney were submitted for aerobic bacterial culture and grew very small numbers of mixed growth including Streptococcus equi subsp. zooepidemicus, Streptococcus viridans, Micrococcus sp. and 2 colonies each two types of Actinobacillus sp.
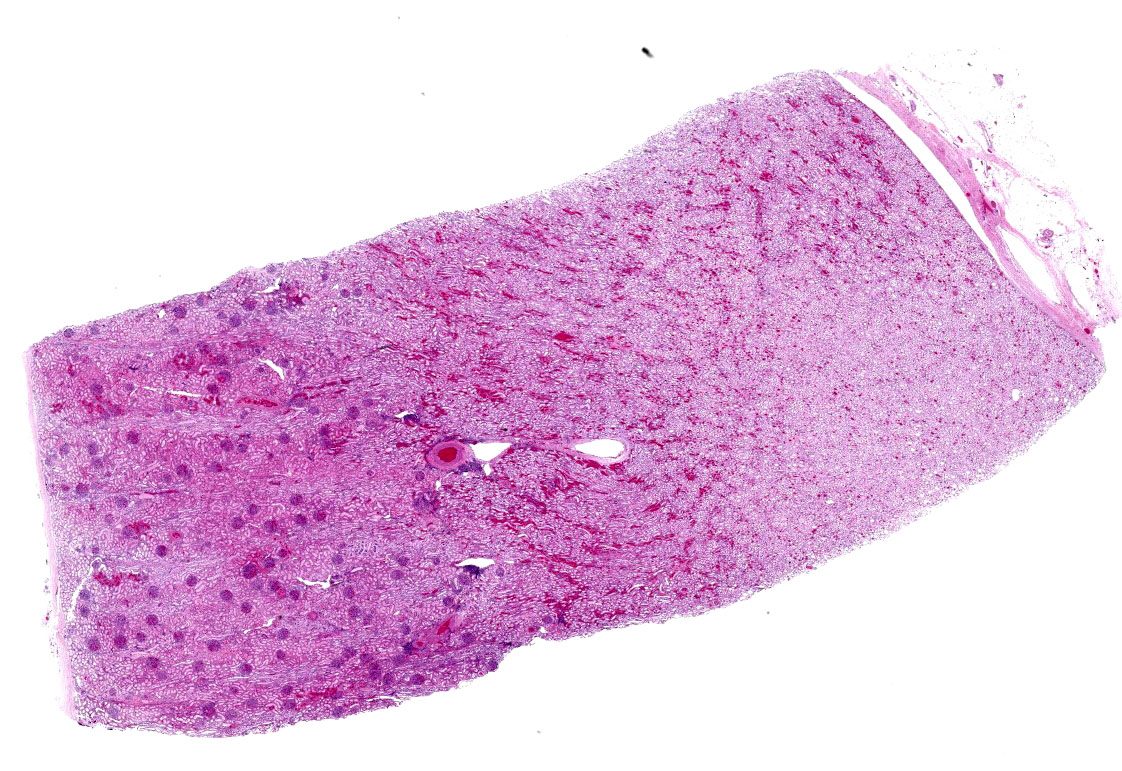
Microscopic description:
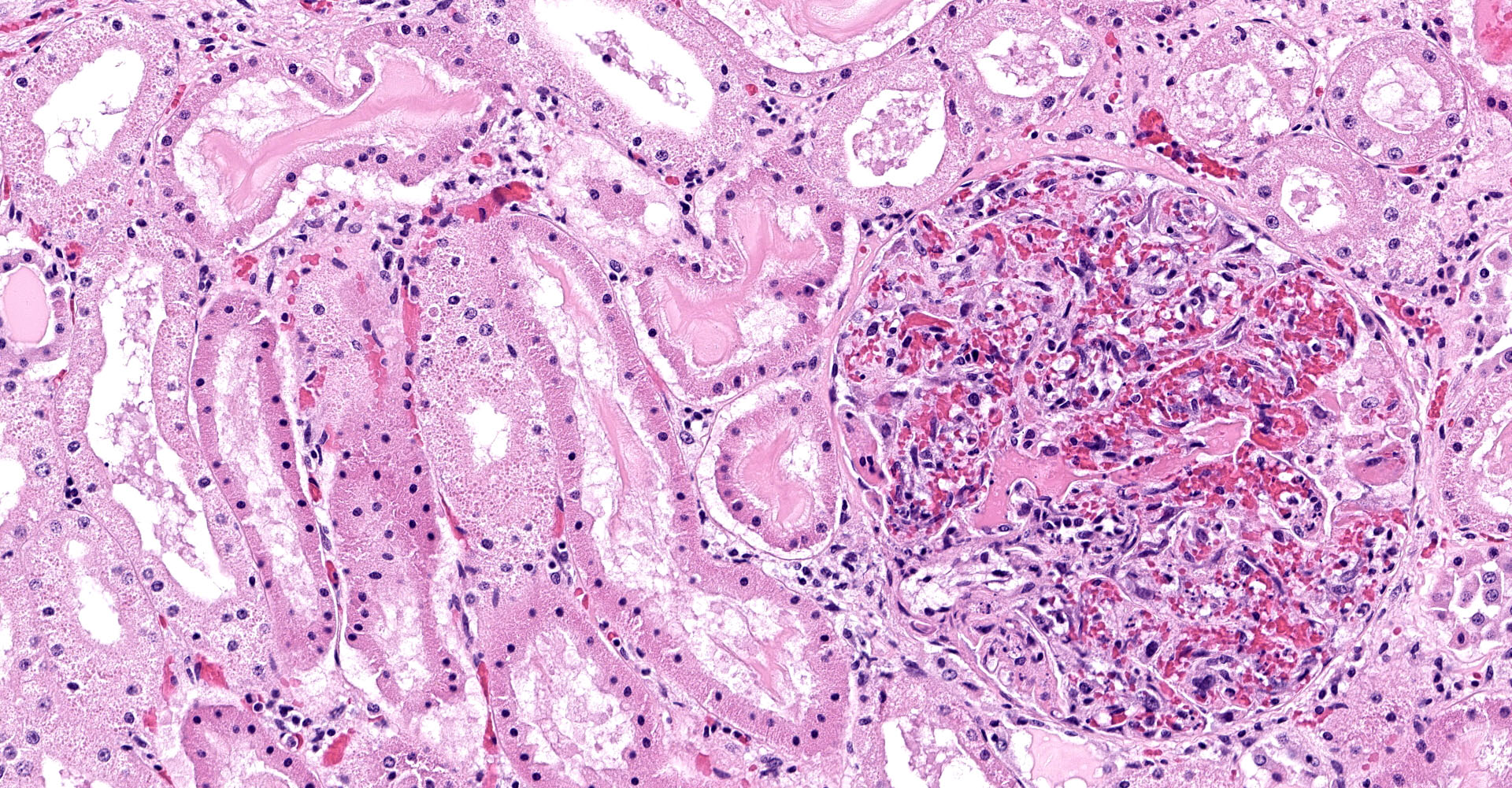
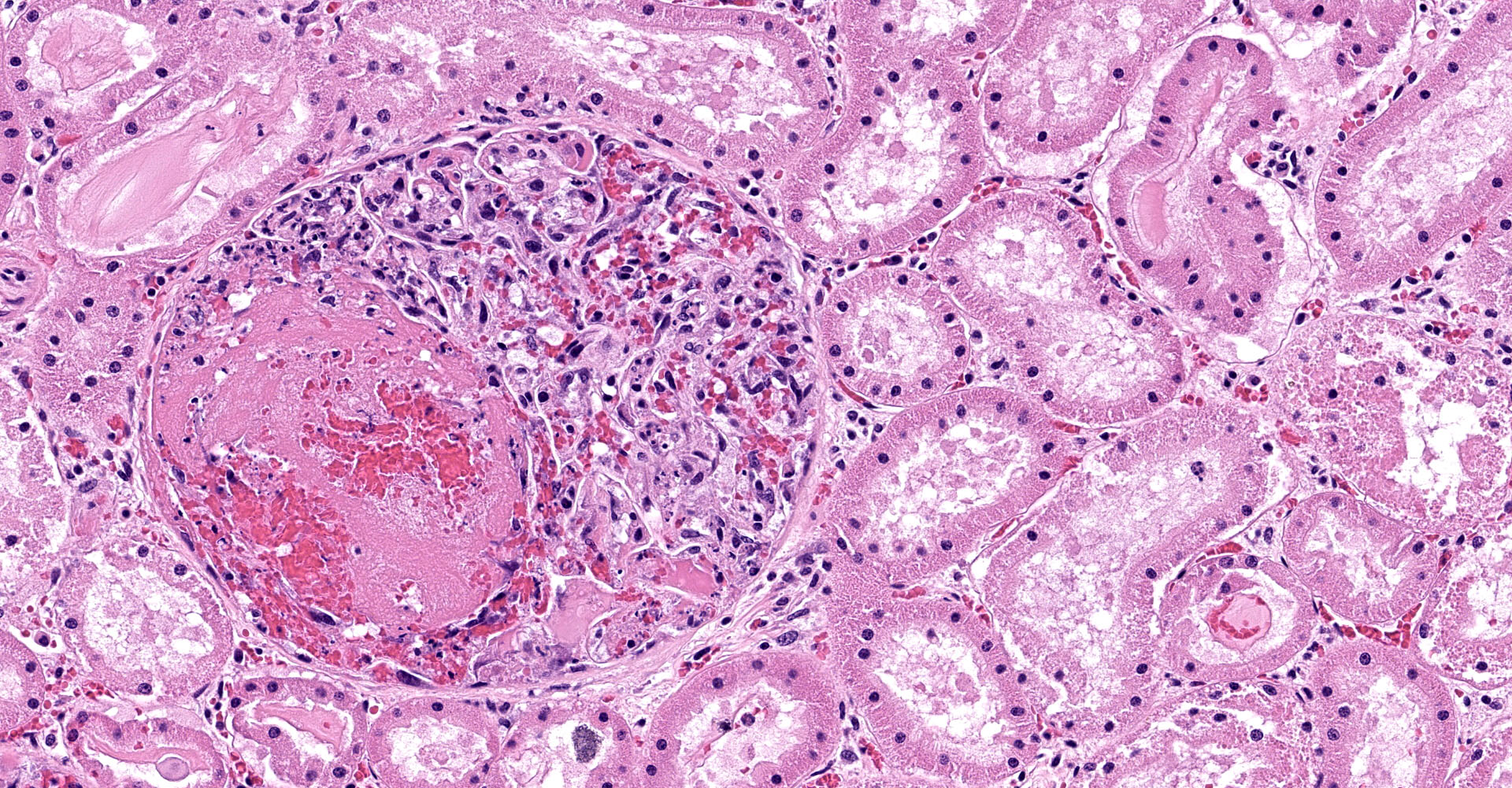
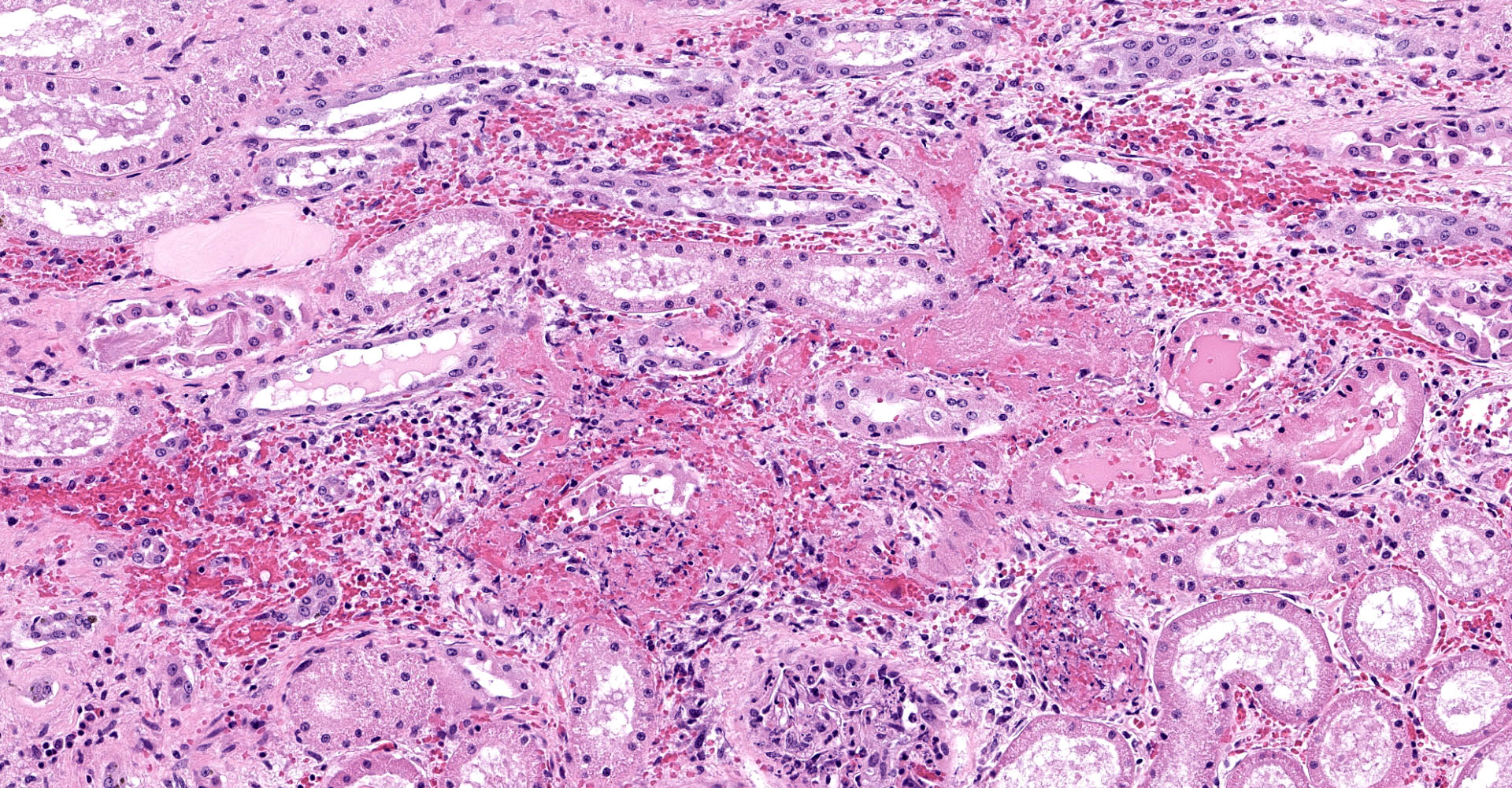
One section of kidney is examined in which glomeruli are diffusely and globally enlarged and hypercellular with variable expansion and vacuolation of the mesangial matrix. Capillary lumina are frequently obstructed by swollen endothelial cells, thickened basement membranes and inflammatory cells predominantly composed of mononuclear cells and neutrophils. Segmental necrosis is prominent throughout glomeruli with glomerular tufts and capillary walls being replaced by amorphous eosinophilic material (fibrin) admixed with nuclear fragments (karyolysis). Glomeruli tufts are occasionally shrunken, eosinophilic and hypocellular (obsolescence). Tubules are ectatic with the lumina often containing homogenous to finely granular eosinophilic material (protein), red blood cells, or sloughed epithelial cells. Tubular epithelium demonstrates one of the following changes: attenuation and flattening, hypereosinophilic epithelial cytoplasm with pyknotic nuclei (individual cell necrosis), microvacuolated cytoplasm, and slightly basophilic cytoplasm with vesiculate nuclei (regeneration). Multifocally tubules are replaced by fibrosis or separated by clear spaces (suggestive of edema) and hemorrhage. The perivascular interstitium is multifocally infiltrated by small aggregates of lymphocytes and plasma cells.
Special stains:
Kidney (H&E - 2-micron section): The glomeruli are hypercellular with scattered nuclear debris. Scant inflammatory cells (neutrophils) are noted within the affected glomeruli. There is also multifocal peri-glomerular fibrosis and multifocal interstitial lymphoplasmacytic inflammation. Occasionally, there are obsolescent glomeruli. Multifocally, renal tubules have attenuation and simplification of the renal tubular epithelium.
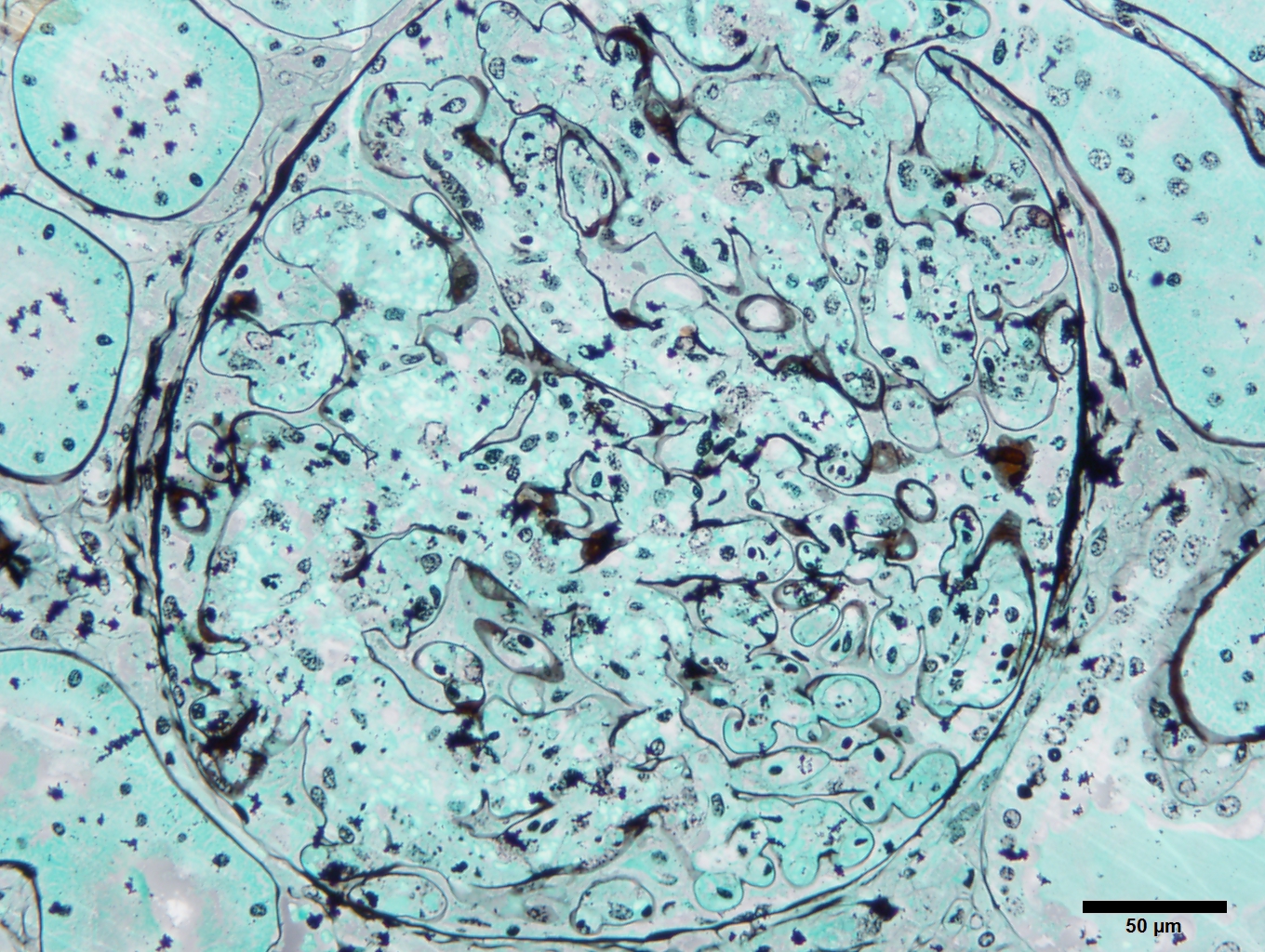
Kidney (Periodic acid-Schiff - 2-micron section): The glomeruli are hypercellular but the glomerular basement membrane appears unremarkable. Mesangial lysis is present in many glomeruli. Multifocally, the renal tubules are filled with protein droplets indicating protein leakage through the filtration barrier.
Kidney (Trichrome ? 2-micron section): Glomerular fuschinophilic deposits are not observed.
Kidney (Jones Methenamine Silver Stain - 2-micron section): The presence of mesangial lysis is accentuated.
Kidney (PTAH): Intra-glomerular fibrin associated with hemorrhage is present within the glomerular tuft.
Immunohistochemistry:
Kidney (CD204): There were many CD204 positive macrophages within the affected glomeruli. This finding may be indicative of an immune response to mesangiolysis.
Kidney (Smooth Muscle Actin): Parietal cells within affected glomeruli had positive immunoreactivity for smooth muscle actin. This finding has been noted in different types of renal injury such as glomerulonephritis.
Contributor's morphologic diagnosis:
1. Kidney (light microscopy): Severe, diffuse, global, acute proliferate glomerulonephritis with mesangial lysis, hemorrhage, segmental glomerular necrosis, tubular epithelial attenuation, necrosis, regeneration and loss, and protein and cellular casts.
2. Kidney (TEM diagnosis): Severe, global, acute immune complex glomerulonephritis (ICGN) with subepithelial, intramembranous, subendothelial and peri-mesangial dense deposits.
3. Kidney: Mild, multifocal, chronic lymphoplasmacytic interstitial nephritis with fibrosis and focal, diffuse, glomerular obsolescence.
Contributor's comment:
Etiologic agents and exact pathogenesis of immune-complex glomerulonephritis (ICGN) in animals are not well understood. It has been postulated that infectious diseases may provoke immune complex deposition within glomeruli resulting in glomerular pathology.8 In horses, equine infectious anemia (EIA), Streptococcus- and herpesvirus antigen-antibody complexes have previously been documented to cause renal failure resulting from glomerulonephritis.2,5 In humans, post-streptococcal glomerulonephritis (PSGN) is caused by prior infection with nephritogenic strains of beta-hemolytic streptococcus.
Human PSGN is associated with a previous skin, throat, or respiratory infection by group A streptococcus (Streptococcus pyogenes), albeit rare reports of groups C or G streptococcus are reported.11 An epidemic outbreak in Nova Serrana, a small rural Brazilian community humans was associated with Streptococcus zooepidemicus transmitted during ingestions of unpasteurized, mastitic, cow milk.1 Streptococcus equi subspecies zooepidemicus (S. zooepidemicus) is a ?-hemolytic, Lancefield group C streptococcal bacterium.3
S. zooepidemicus is known to be a commensal, opportunistic pathogen in horses associated with inflammatory airway disease in Thoroughbred racehorses17,18, uterine infections in mares9,13 and ulcerative keratitis4. Renal lesions develop as a consequence of either deposition of circulating antigen-antibody complexes or from in situ reactions to streptococcal antigens. Historically implicated S. zooepidemicus antigens include M protein, R antigen, and a common protein antigen (CSCA) of S. equi and S. zooepidemicus.6,14 Recent studies on gene expression of nephritis associated plasmin receptor (NAPlr, a glyceraldehyde 3-phosphate dehydrogenase), the streptococcal pyrogenic exotoxin (erythrotoxin) B (SPEB), and the SPEB zymogen precursor (zSPEG) in several streptococcal strains highlight the growing importance of these nephritogenic antigens.11
Light microscopic findings in post-streptococcal glomerulonephritis typically shows diffuse proliferative and exudative glomerulonephritis (GN). Glomeruli are enlarged with global endocapillary hypercellularity and variable numbers of neutrophils. With time, the endocapillary hypercellularity may transitions into a predominantly mesangial hypercellularity. Rarely do cases demonstrate membranoproliferative GN (MPGN). In humans, 60% of cases had histologic evidence of acute tubular injury.10 Immunofluorescence microscopy has been extensively studied in people demonstrating that in the first 2-3 weeks, C3 and IgG deposits in the capillary walls and mesangial areas in a finely granular, "starry sky" pattern. With time, there is a predominance of C3 deposition in the mesangium, including subepithelial deposits within the mesangial "waist". Coarse to confluent granular staining along the glomerular capillary walls ("garland" pattern) creates the subepithelial "humps" that are the characteristic ultrastructural feature of this disease. These can be seen early or late in the disease.11 Immunofluorescence was not pursued in this case due to the lack of available equine antibodies.
Other etiologic agents were considered in this case due to the light microscopic findings. A comprehensive equine upper respiratory panel and an equine infectious anemia ELISA were performed to rule out herpesvirus and EIA. A toxic glomerular vasculopathy from envenomation was also deliberated due to the dilated and ruptured capillaries within glomeruli (mesangiolysis and capillary ballooning) with hyaline casts, in addition to the echinocytosis. Echinocytes (crenated erythrocytes) may be observed after envenomation, presumably secondary to the action of venom phospholipases; however, in horses, echinocytosis has been demonstrated with systemic electrolyte depletion (endurance exercise, furosemide treatment, diarrhea, and systemic disease).7 Ultimately, dense deposits discounted this differential since dense deposits should not be present in a typical case of envenomation. Demonstration of IgG and C3 deposition in the renal glomerulus could provide confirmatory evidence that the glomerular pathology is due to an immune response. Regardless, pulmonary and renal culture results are highly suggestive of a post-streptococcal glomerulonephritis.
Contributing Institution:
University of California, Davis
https://www.vetmed.ucdavis.edu/hospital/support-services/lab-services/anatomic-pathology-service
JPC diagnosis:
Kidney: Glomerulonephritis, proliferative and with superimposed microangiopathy, diffuse, severe, with tubular degeneration, necrosis, and regeneration, proteinosis, and red cell casts.
JPC comment:
The contributor provides a concise summary of immune-complex glomerulonephritis. In addition to post-streptococcal glomerulonephritis, the most common causes of type III hypersensitivity reactions include serum sickness, systemic lupus erythematosus, farmer's lung (hypersensitivity pneumonitis), and rheumatoid arthritis. Common to all of these etiologies is a similar three step process that leads to tissue damage. First, endogenous or exogenous antigen triggers antibody formation, usually within 4-10 days. Antibodies bind antigen and form circulating complexes. Second, when there is a slight excess of antigen, the formed immune complexes are smaller and filter out of circulation more easily, typically in tissues where a filtrate is formed from blood, like synovial fluid or urine. This explains how joints and glomeruli are primary targets of immune complex deposition. Thirdly, the classical pathway to complement activation leads to the release of C3a and C5a (anaphylatoxins) that recruit neutrophils and macrophages which cause inflammatory damage to tissues.16
Specific to the proposed pathogenesis of this case, Streptococcus equi subsp zooepidemicus has a number of identified fibrinonectin binding proteins (FNZ/FNE, FNZ2/FNEB, SFS), M-like proteins (SzM/SeM, SzPSe/SzP), immunoglobulin binding proteins (ZAG), toxins (streptolysin S), and a number of superantigens (SpeK, SpeL, SzeN, SzeP, SzeF). The combination of superantigens and immunoglobulin binding proteins make this bacterium a potent source of circulating immune complexes.15
Human medicine has a long history of documenting acute post-streptococcal glomerulonephritis (APSGN), but usually caused by a group A b-haemolytic Streptococcal infection. Starting in the mid-1900's, the incidence of APSGN cases began to decline, attributed to improved standards of living and socioeconomic conditions in most developed countries. However, a recent surge of methicillin-resistant Staphyloccocus aureus infections over the last 20 years has changed the epidemiology of post-infection glomerulonephritides. While the number of veterinary cases of immune complex glomerulonephritis attributed to Staphylococcus aureus is unknown in large part, the shift observed in human clinical medicine may portend a shift in our patients as well.12
A portion of the conference discussion centered on the capillary thrombi seen in glomeruli. Evaluated together with the TEM images revealing extensive endothelial damage, this consensus was that this horse had a superimposed thrombotic microangiopathy (TMA), affecting the glomerular and peritubular capillaries.
Leptospirosis was discussed as a possible differential diagnosis in this case. The moderator more often sees tubulocentric damage associated with leptospira, but pathologists located in different regions of the United States have seen glomerular-centric cases of equine leptospirosis, which also lead to glomerular microthrombosis.
References:
1. Baiter S, Benin A, Pinto SW, Teixeira LM, Alvim GG, Luna E, Jackson D, LaClaire L, Elliott J, Facklam R, Schuchat A. Epidemic nephritis in Nova Serrana, Brazil. The Lancet. 2000 May 20;355(9217):1776-80.
2. Banks KL, Henson JB, McGuire TC. Immunologically mediated glomerulitis of horses. I. Pathogenesis in persistent infection by equine infectious anemia virus. Laboratory investigation; a journal of technical methods and pathology. 1972 Jun;26(6):701.
3. Barnham M, Thornton T, Lange K. Nephritis caused by Streptococcus zooepidemicus (Lancefield group C). The Lancet. 1983 Apr 30;321(8331):945-8.
4. Brooks DE, Andrew SE, Biros DJ, Denis HM, Cutler TJ, Strubbe DT, Gelatt KN. Ulcerative keratitis caused by beta?hemolytic Streptococcus equi in 11 horses. Veterinary ophthalmology. 2000 Jun;3(2?3):121-5.
5. Cianciolo RE, Mohr FC. Urinary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:401-413.
6. Cu GA, Mezzano S, Bannan JD, Zabriskie JB. Immunohistochemical and serological evidence for the role of streptococcal proteinase in acute post-streptococcal glomerulonephritis. Kidney Int 1998; 54:819.
7. Geor RJ, Lund EM, Weiss DJ. Echinocytosis in horses: 54 cases (1990). Journal of the American Veterinary Medical Association. 1993 Mar;202(6):976-80.
8. Heptinstall RH. Heptinstall's Pathology of the Kidney. Lippincott Williams & Wilkins; 2007.
9. Hong CB, Donahue JM, Giles Jr RC, Petrites-Murphy MB, Poonacha KB, Roberts AW, Smith BJ, Tramontin RR, Tuttle PA, Swerczek TW. Etiology and pathology of equine placentitis. Journal of Veterinary Diagnostic Investigation. 1993 Jan;5(1):56-63.
10. Nasr SH, Markowitz GS, Stokes MB, Said SM, Valeri AM, D'Agati VD. Acute postinfectious glomerulonephritis in the modern era: experience with 86 adults and review of the literature. Medicine. 2008 Jan 1;87(1):21-32.
11. Rodriguez-Iturbe B, Haas M. Post-streptococcal glomerulonephritis. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet] 2016 Feb 10. University of Oklahoma Health Sciences Center.
12. Satoskar AA, Parikh SV, Nadasdy T. Epidemiology, pathogenesis, treatment and outcomes of infection-associated glomerulonephritis. Nature Reviews Nephrology. 2020. 16:32-50.
13. Smith KC, Blunden AS, Whitwell KE, Dunn KA, Wales AD. A survey of equine abortion, stillbirth and neonatal death in the UK from 1988 to 1997. Equine veterinary journal. 2003 Jul;35(5):496-501.
14. Timoney JF, Trachman JU. Immunologically reactive proteins of Streptococcus equi. Infection and immunity. 1985 Apr 1;48(1):29-34.
15. Turner CE, Bubba L, Efstratiou A. Pathogenicity factors in Group C and G Streptococci. Microbiology Spectrum. 2019;7(3):doi:10.1128/microbiolspec.GPP3-0020-2018.
16. Usman N, Annamaraju P. Type III hypersensitivity reaction. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2020.
17. Wood JL, Burrell MH, Roberts CA, Chanter N, Shaw Y. Streptococci and Pasteurella spp. associated with disease of the equine lower respiratory tract. Equine veterinary journal. 1993 Jul;25(4):314-8.
18. Wood JL, Newton JR, Chanter N, Mumford JA. Association between respiratory disease and bacterial and viral infections in British racehorses. Journal of clinical microbiology. 2005 Jan 1;43(1):120-6.