WSC 22-23
Conference 2
CASE III: 672918 User 8 (JPC 4166546)
Signalment:
Tissue from a 9-year-old neutered female German Shepherd dog (Canis lupus familiaris)
History:
The patient was presented for bilateral hind limb ataxia. MRI showed an intramedullary spinal cord lesion at L 4-5. Additional masses on the body wall found with ultrasound. Specimens of spleen and mammary gland samples were submitted in addition to spinal cord, and were diagnosed as nodular lymphoid hyperplasia and benign mixed mammary tumor (not shown).
Gross Pathology:
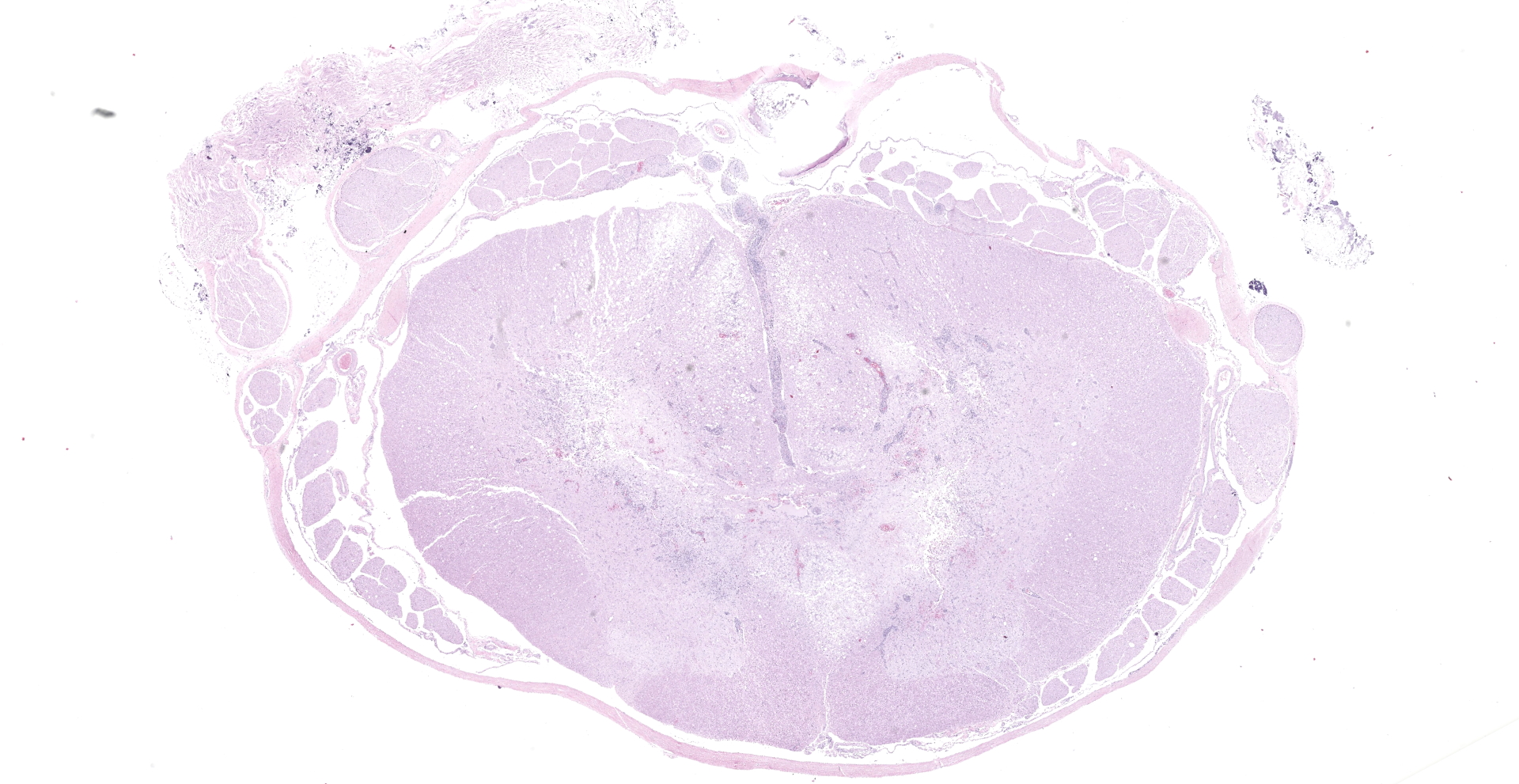
Several cross-sections of lumbar spinal cord contained soft, reddish brown, tissue affecting the gray matter bilaterally.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
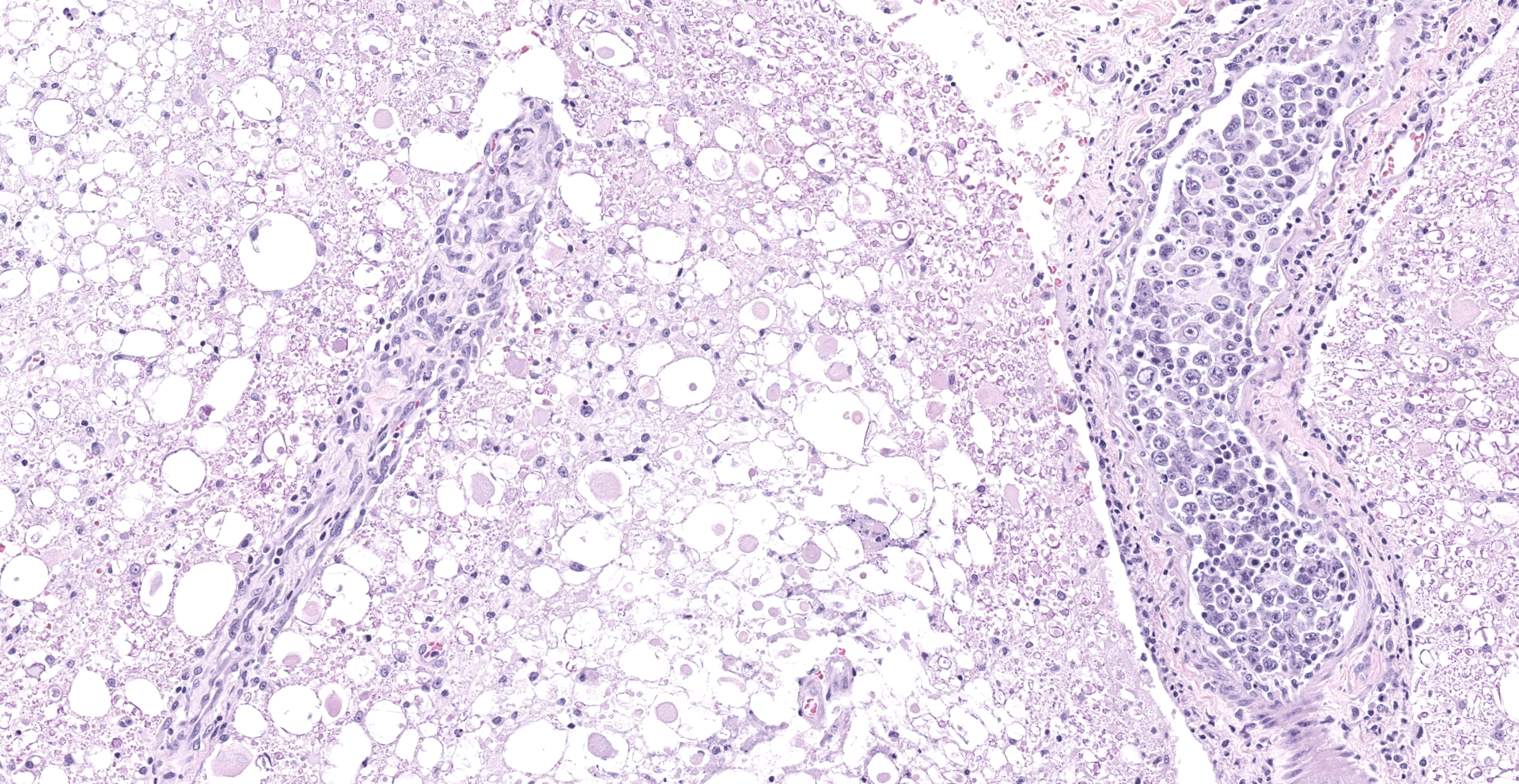
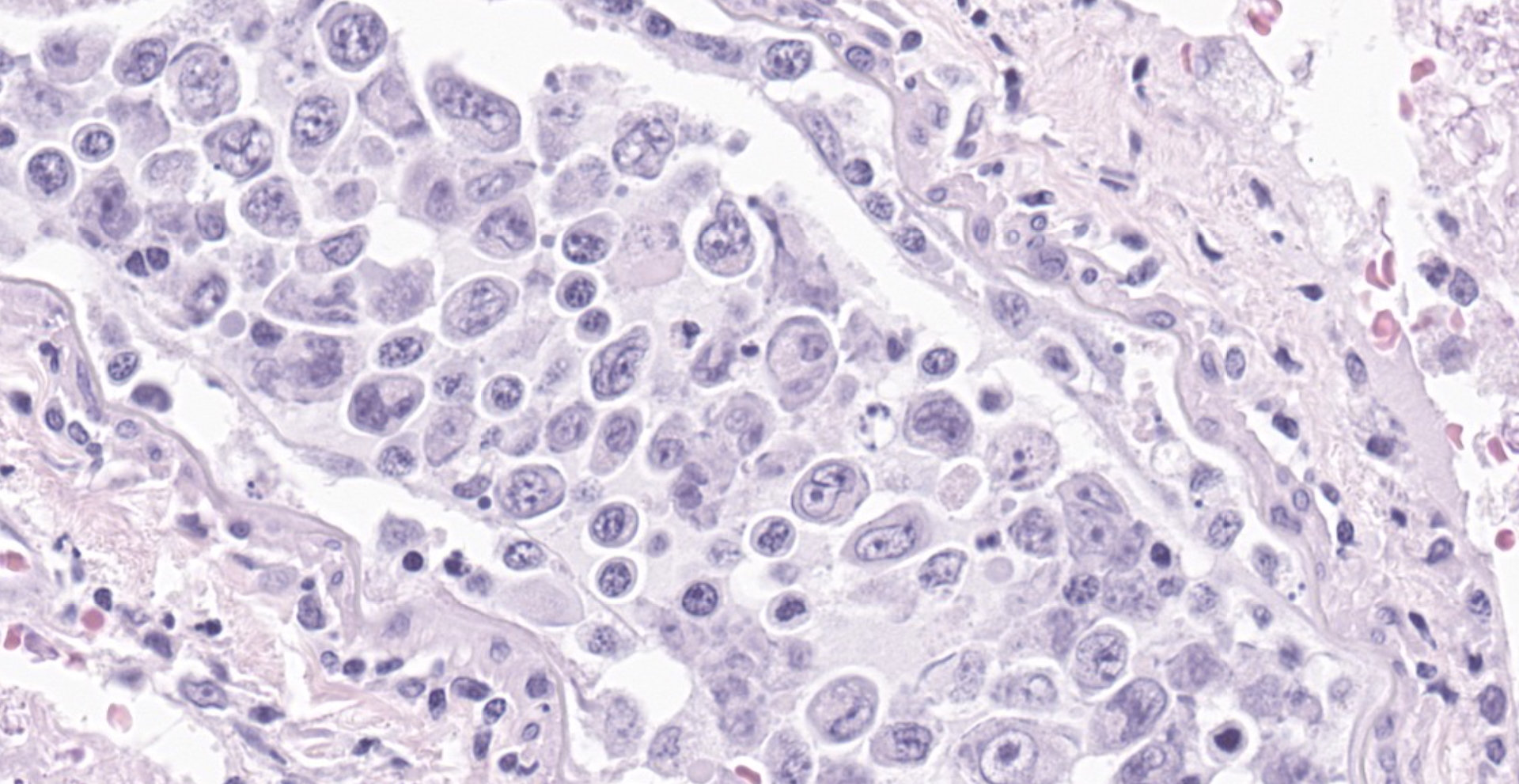
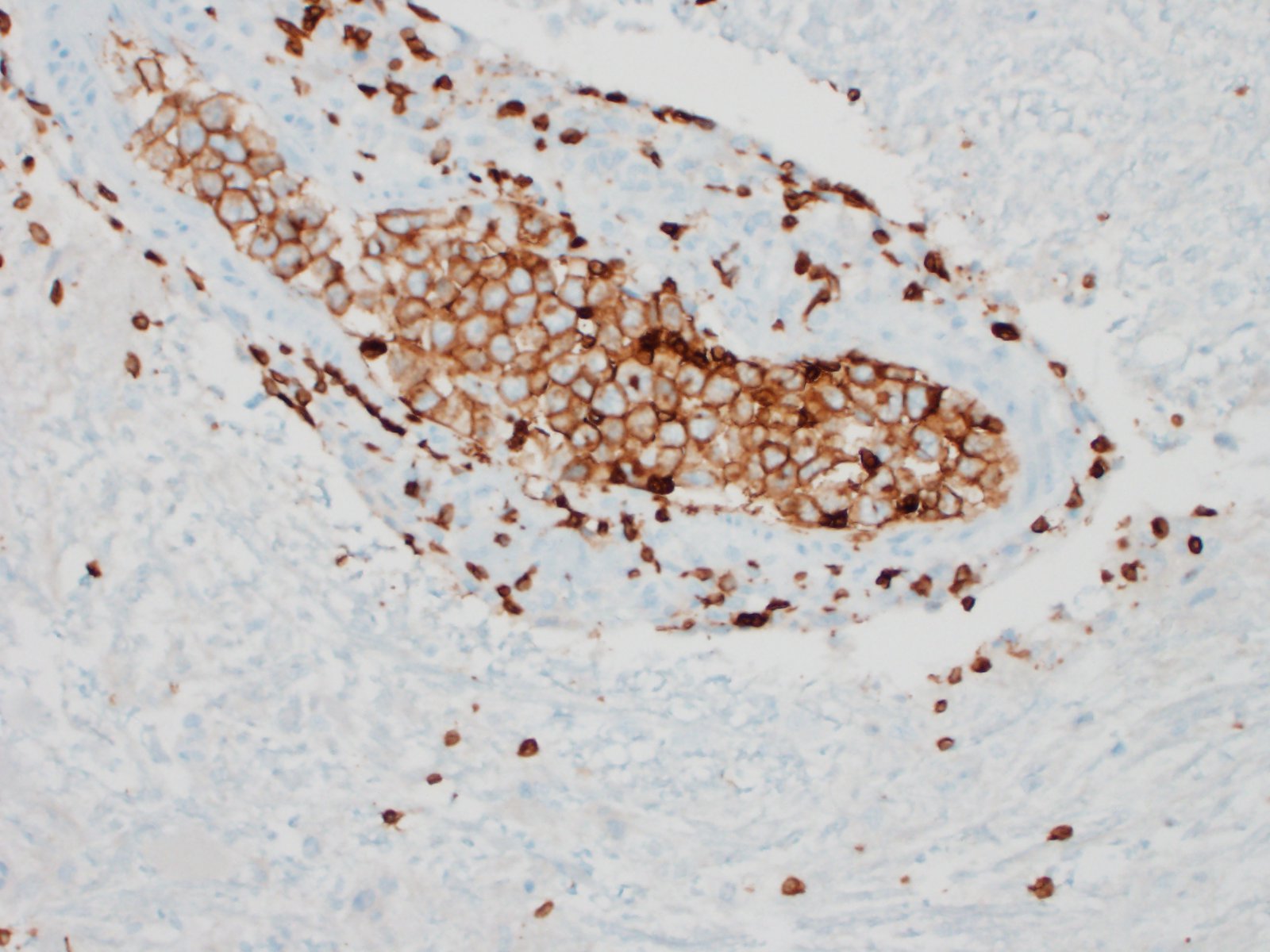
Much of the spinal cord gray matter and the ventrolateral white matter are soft and malacic. The tissue architecture is obliterated, with endothelial hypertrophy and hyperplasia of remaining blood vessels. Gitter cells fill some of the tissue spaces. The ventral spinal artery is obliterated by a large lumenal thrombus. It and the arteries extending up along the ventral median fissure are blocked by large round cells. Wallerian degeneration affected much of the ventral white matter. Cells are characterized by scant amphophilic cytoplasm and a large cleaved to reniform nuclei. The chromatin is arranged in coarse, irregular clumps; nuclear outlines are very irregular. Intravascular mitoses are present in the population. These cells are not present in smaller blood vessels. A less severely affected section of spinal cord has ventral white matter Wallerian degeneration, with a smaller ventral spinal artery filled with organisms. Two of the spinal nerves are degenerate and inflamed, with several radicular vessels being blocked (not shown). Lymphoplasmacytic inflammation occurs in the meninges and in perivascular cuffs near malacic spinal cord (also in affected roots). Intravascular tumor cells are immunohistochemically CD3 positive, demonstrated along cell membranes.
Contributor's Morphologic Diagnoses:
- Myelomalacia with ventral spinal and radicular artery tumor embolism
- Intravascular lymphoma
Contributor's Comment:
At first glance the distribution of the spinal cord lesions brings to mind fibrocartilaginous embolism (FCE), due to the commonality of affected blood vessels (primarily the ventral spinal artery and branches, with infarction of the spinal gray matter.3,6 However, both arteries and veins have been reported affected in FCE, while arteries and arterioles are affected in this case.
The presence of neoplastic cells in the ventral spinal artery and its branches is consistent with a diagnosis of large cell lymphoma, a tumor restricted to growth in the lumens of small- to intermediate-sized vessels.7 This tumor would be described as the so-called classic form, with involvement primarily in the organ of presentation (usually brain or skin). A hemophagocytic syndrome-associated form can also occur in which patients present with multi-organ failure.12 In humans, neoplastic cells express B cell markers (such as CD20 or CD79a). T-cell tumors are much less common,14 but do occur. In people, neoplastic growth can occasionally extend across vascular walls, as in a few areas in this dog, and can be mistaken for primary parenchymal lymphoma. T cell intravascular lymphomas are rare in humans and have been reported in dogs.2,5,11 A tally of immunohistochemistry of intravascular lymphomas in 2 case series 2,5 revealed 11 T cell, 4 B cell and 10 Non-T non-B cell tumors. Most human tumor are of B cell origin.7
The intravascular location of tumor cells leads to thrombosis and infarction, which are responsible for clinical signs. Intravascular B cell lymphomas in people lack expression of surface adhesion molecules (e.g. cd29 and ICAM 1). Tumor cells proliferate within the blood vessels without being able to exit them, potentially occluding them and causing ischemia without tissue invasion. Cells also lack the expression of matrix metalloproteinase (MMP) 2 and MMP9 involved in the extravascular invasion of other lymphomas. Therefore, intravascular growth is associated with the inability of intravascular lymphoma cells to infiltrate extravascular tissues.12
Contributing Institution:
University of Missouri
JPC Diagnosis:
- Spinal cord, meningeal and parenchymal vessels: Intravascular lymphoma.
- Spinal cord: Myelomalacia, multifocal to coalescing, moderate to marked, with thrombosis.
JPC Comment:
Historically, intravascular lymphoma in humans was known by the name malignant angioendotheliomatosis due to the unique histologic appearance mimicking proliferation of endothelial cells; however, clinical, immunohistochemical, ultrastructural, and therapeutic studies revealed the disease to be an angiotropic form of lymphoma.4In 1988, a case of angioendotheliomatosis in an adult German shorthaired pointer was also confirmed to be angiotropic lymphoma through ultrastructural and immunofluorescence investigation.4 Since then, this disease has been reported rarely in dogs, and even more rarely in cats and horses.10
Intravascular lymphoma most frequently affects the central nervous system and is characterized by rapidly progressive neurologic signs secondary to infarction with death occurring within weeks of initial clinical signs. Diagnosis is always made post-mortem. Rarely, neoplastic cells have been observed ante-mortem on peripheral blood smears or in cavitary effusions; however, even in these cases, refining the diagnosis beyond round cell neoplasm was not possible until post-mortem histopathology.10,13 Differential diagnoses on histopathology include other round cell neoplasms which form a cuff around blood vessels, vaccine reactions, or systemic reactive angioendotheliomatosis.15
As the contributor described, neoplastic lymphocytes appear unable to exit blood vessels in this condition, and studies in human and veterinary patients illustrate altered expression of clusters of differentiation (CD) markers. In humans, neoplastic cells of intravascular lymphoma lack CD11a, 18, and 29 expression.10A study of canine intravascular lymphoma found that there was decreased CD29 expression and increased CD44 expression compared to canine primary/metastatic CNS lymphoma.5 Some of these markers are critical during the leukocyte adhesion cascade and delivery of inflammatory cells to sites of inflammation.
Briefly, the leukocyte adhesion cascade has five steps which are partially propelled by cytokines: margination, rolling, integrin activation, stable adhesion, and transendothelial migration.1,9 Selectins are adhesion molecules which cause marginated leukocytes to slow and begin rolling, and integrins are adhesion molecules which then enforce firm adhesions between endothelial cells and leukocytes. CD11a/18 is the beta-2 integrin LFA-1 which binds intercellular adhesion molecules (ICAM) 1 and 2 (CD 54 and 102, respectively) on the endothelium during stable adhesion.9 CD29 is found in beta-1 integrins (such as VLA-4) which facilitate leukocyte adhesion and transendothelial migration.1,9 The previously noted alterations in expression of these markers in intravascular lymphoma provide some insight into the intravascular growth and lack of extravasation seen in this neoplasm.
References:
- Ackermann MR. Inflammation and Healing. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. St. Louis, MO: Mosby Elsevier; 2021:104-170.
- Bush WW, Throop JL, McManus PM, et al. Intravascular lymphoma involving the central and peripheral nervous systems in a dog. J Am Anim Hosp Assoc. 2003;39(1):90-96.
- Cauzinille L, Kornegay JN. Fibrocartilaginous embolism of the spinal cord in dogs: review of 36 histologically confirmed cases and retrospective study of 26 suspected cases. J Vet Intern Med. 1996;10(4):241-245.
- Dargent FJ, Fox LE, Anderson WI. Neoplastic angioendotheliomatosis in a dog: an angiotropic lymphoma. Cornell Vet. 1988; 78(3): 253-62.
- Degl'Innocenti S, Camera ND, Falzone C, et al. Canine Cerebral Intravascular Lymphoma: Neuropathological and Immunohistochemical Findings. Vet Pathol. 2019;56(2):239-243.
- Griffiths IR, Barker J, Palmer AC. Cholesterol masses in association with spinal cord infarction due to intervertebral disc emboli. Acta Neuropathol. 1975 Oct 27;33(1):85-88.
- Grimm KE, O'Malley DP. Aggressive B cell lymphomas in the 2017 revised WHO classification of tumors of hematopoietic and lymphoid tissues. Ann Diagn Pathol. 2019;38:6-10.
- Kawai S, Okuda T, Fukui A, et al. Intravascular Lymphoma Presenting as a Cavernous Sinus Tumor. Intern Med. 2019;58:2085-2089.
- Kuman V, Abbas AK, Aster JC, eds. Inflammation and Repair. In: Robbins & Cotran Pathologic Basis of Disease. 10th Philadelphia, PA: Elsevier, Inc; 2021: 71-114.
- Lane LV, Allison RW, Rizzi TR, et al. Canine intravascular lymphoma with overt leukemia. Veterinary Clinical Pathology. 2012; 41(1): 84-91.
- McDonough SP, Van Winkle TJ, Valentine BA, et al. Clinicopathological and immunophenotypical features of canine intravascular lymphoma (malignant angioendotheliomatosis). J Comp Pathol. 2002; 126(4):277-88.
- Poropatich K, Dittmann D, Chen YH, et al. A Small Case Series of Intravascular Large B-Cell Lymphoma with Unexpected Findings: Subset of Cases with Concomitant Extravascular Central Nervous System (CNS) Involvement Mimicking Primary CNS Lymphoma. J Pathol Transl Med. 2017;51(3):284-291.
- Ridge L, Swinney G. Angiotropic intravascular lymphosarcoma presenting as bi-cavity effusion in a dog. Australian Veterinary Journal. 2204; 82(1): 616-618.
- Setoyama M, Mizoguchi S, Orikawa T, et al. A case of intravascular malignant lymphomatosis (angiotropic large-cell lymphoma) presenting memory T cell phenotype and its expression of adhesion molecules. J Dermatol. 1992;19(5):263-9.
- Valli VE, Bienzle D, Meuten DJ. Tumors of the Hemolymphatic System. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th Ames, IO: John Wiley & Sons, Inc; 2017: 203-321.