Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 7
October 17, 2018
CASE II: 16RD2093 (JPC 4102435-00).
Signalment: 8-year-old male neutered Yorkshire terrier dog, Canis familiaris
History: The patient presented to a veterinary ophthalmologist and was diagnosed with a blind right eye (OD) with retinal detachment and potentially an opaque structure posterior to the lens with areas of hemorrhage. The right eye was enucleated, formalin-fixed, and submitted for histopathology.
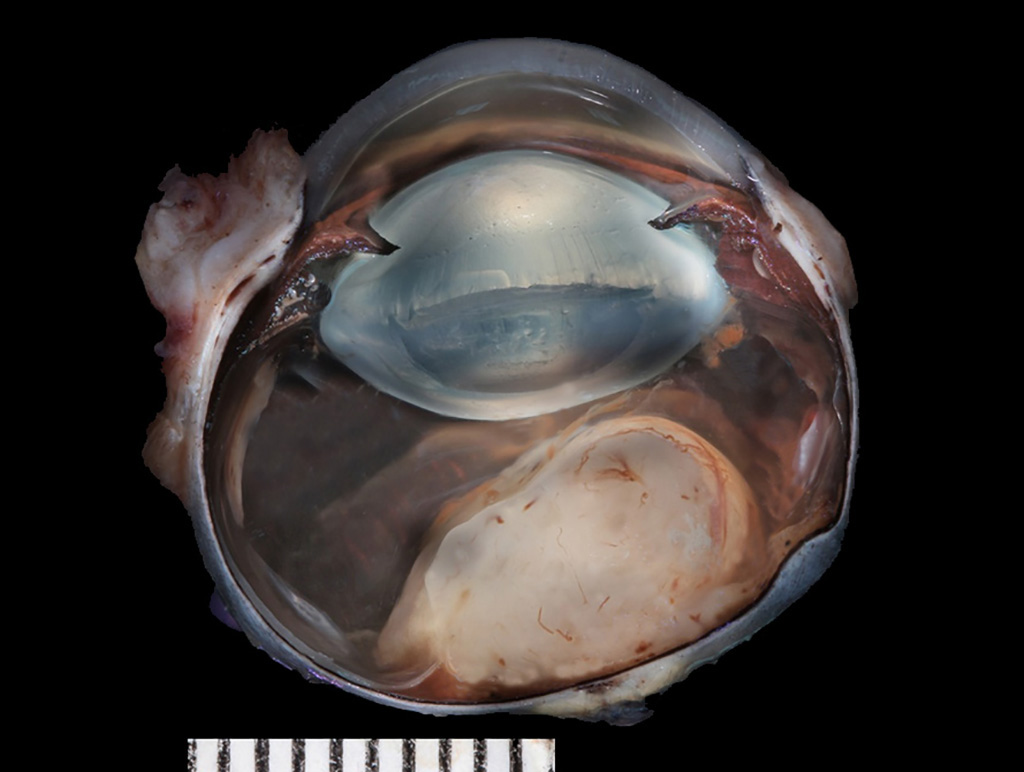
Gross Pathology: The formalin-fixed left eye was section at the dorsoventral plane. There was a soft, tan and homogeneous mass adjacent to the optic nerve protruding into the vitreous.
Laboratory results: Ocular ultrasound revealed a soft tissue opacity extending from the optic nerve into the vitreal space.
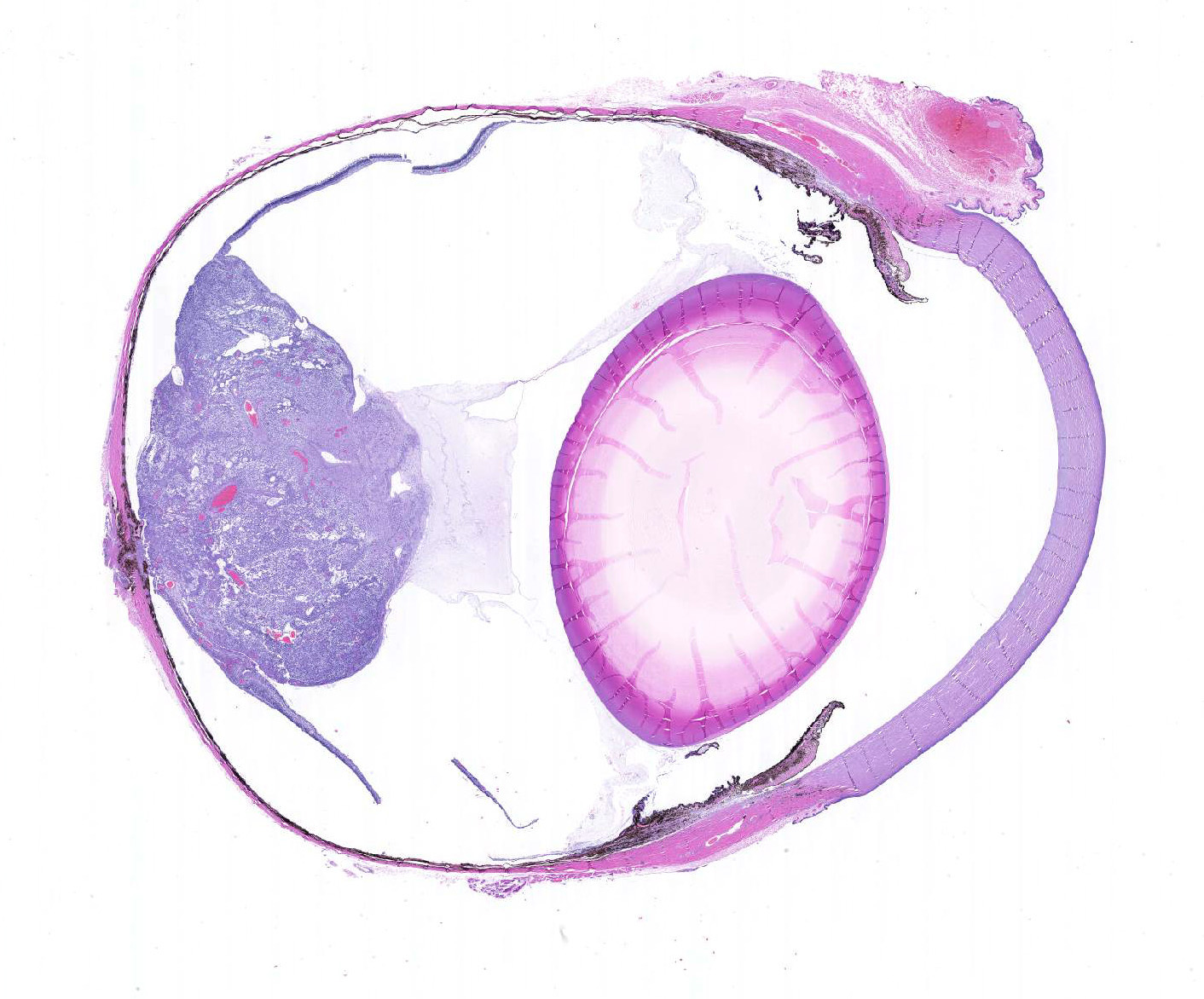
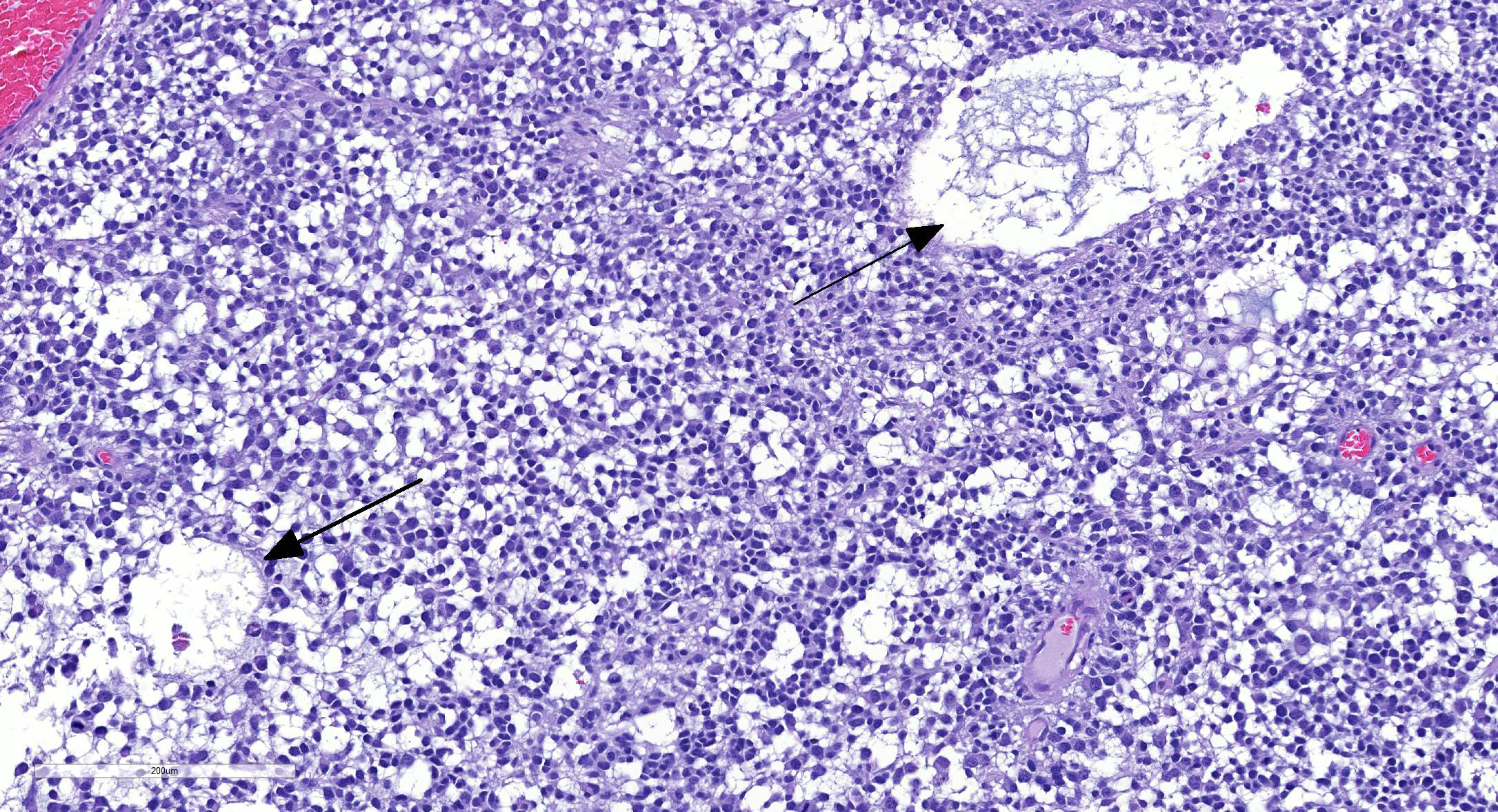
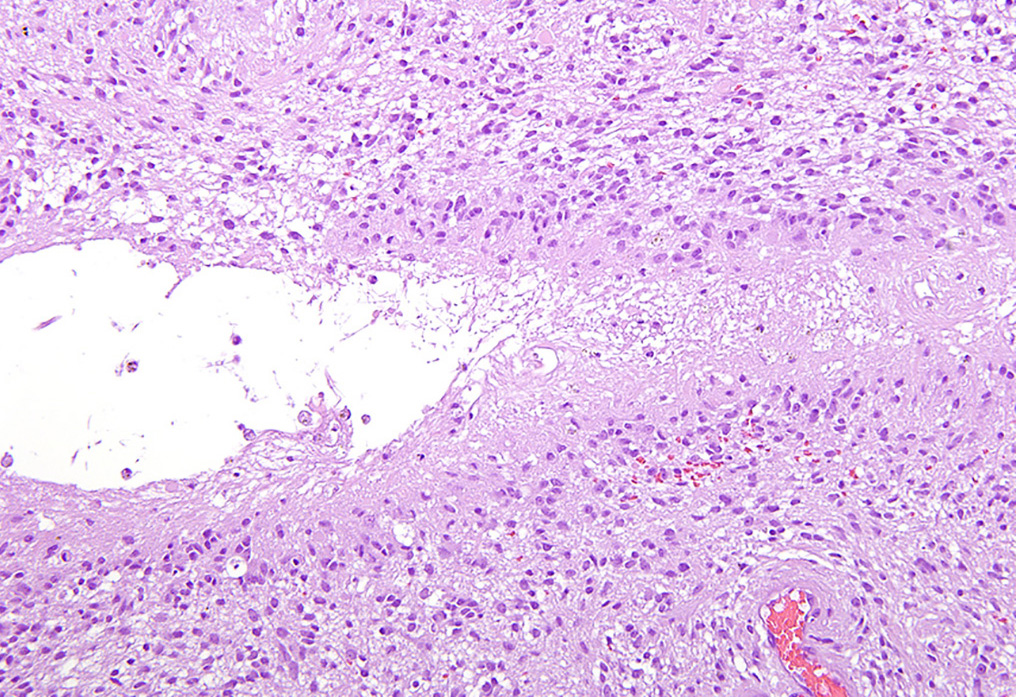
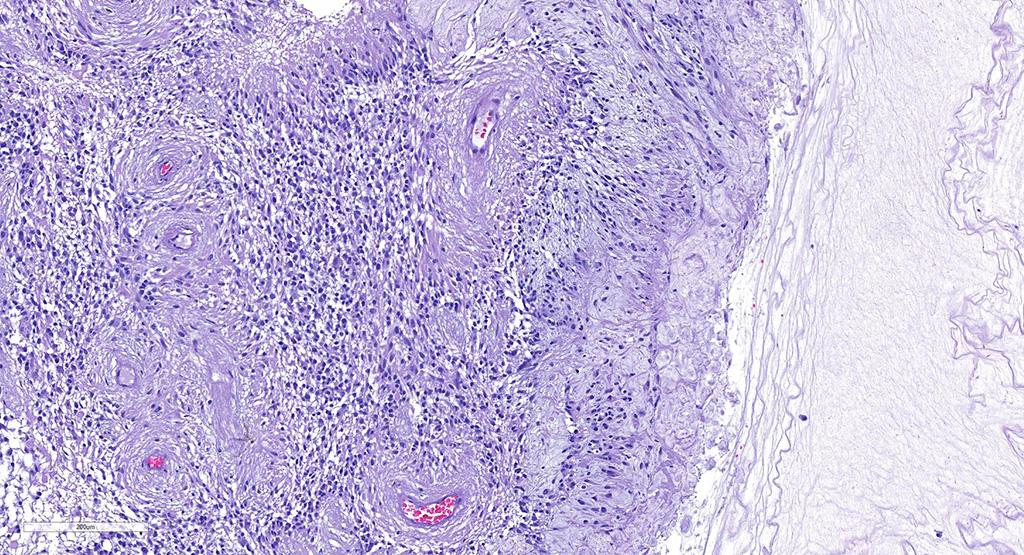
Microscopic Description: There is an unencapsulated, well-delineated and densely cellular neoplastic mass infiltrating, expanding and partially effacing the optic nerve head and central retina, protruding into the vitreous. The mass is composed of a mixture of polygonal, spindle and round cells arranged in short interlacing streams and bundles supported by a moderately dense fibrovascular stroma. The neoplastic cells have indistinct cell borders, a variable amount of pale eosinophilic fibrillar cytoplasm, oval to elongate nuclei with finely stippled chromatin and 1-3 variably distinct nucleoli. A subset of neoplastic cells presents vacuolated cytoplasm with round and eccentrically located nuclei. Anisocytosis and anisokaryosis are marked; with moderate numbers of karyomegalic cells and a few bi and multinucleated cells. There are 6 mitotic figures in 10 HPFs. There are multifocal to focally extensive areas of necrosis, characterized by tissue hypereosinophilia and accumulation of karyorrhectic debris that are surrounded by palisading neoplastic cells (not in all sections . There are multifocal cavitated areas filled with light amphophilic material thought the mass. At the periphery of the neoplasm and in the inner aspects of the retina adjacent to the mass there is prominent capillary proliferation with occasional formation of glomerular-like tufts. There is a mild accumulation of red blood cells in the anterior vitreous. The retina is diffusely detached and there is moderate hypertrophy of the RPE cells and mild subretinal hemorrhage. The detached retina presents diffuse loss of ganglion cells, atrophy of the inner nuclear layer, and mild and multifocal hemorrhage. The post-laminar portion of the optic nerve (not present in all slides) is markedly atrophied and presents increased numbers of non-neoplastic glial cells (gliosis).
Contributor’s Morphologic Diagnoses:
Left eye:
- Optic nerve and retinal glioma/astrocytoma, high-grade.
- Retina, diffuse retinal detachment with inner retinal atrophy.
- Post-laminar optic nerve, moderate and diffuse atrophy and gliosis.
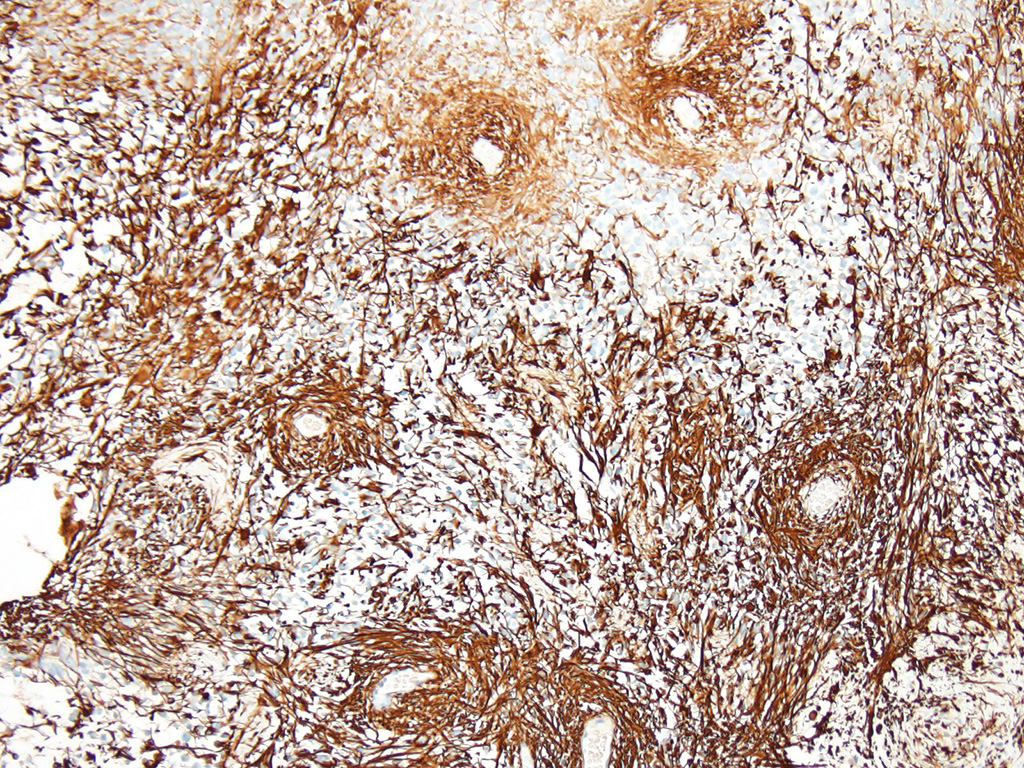
Additional results: Neoplastic cells present strong immunohistochemichal cytoplasmic positivity for glial fibrillary acidic protein (GFAP).
Contributor’s Comment: Gliomas are among the most common primary tumors of the CNS in dogs, their prevalence being only exceeded by meningiomas.4 Based on the World Health Organization (WHO) classification for domestic animal tumors,6 which, in turn, is based on classification of human neoplasms, these tumors are classified according to their cell of origin into astrocytomas, oligodendrogliomas, mixed tumors (oligoastrocytomas), and ependymomas. According to their cytologic characteristics, astrocytomas are further classified into low-grade or well-differentiated, medium-grade or anaplastic, and high-grade or glioblastoma. Low-grade astrocytomas are classified as fibrillary, protoplasmic, or gemistocytic.4,6
Optic nerve and retinal glioma are rare neoplasms that have been sporadically reported in dogs and cats.2,3,9 These tumors can originate and infiltrate the retina, optic nerve head and the post-laminar optic nerve. Most optic nerve and retinal gliomas present morphologic features similar to astrocytomas as seen in other areas of the central nervous system, but rarely these tumors can resemble oligodendrogliomas or present a mixture of astrocytoma and oligodendroglioma features.3,9
Optic nerve/retinal gliomas with predominantly astrocytic phenotype present immunohistochemical positivity for glial fibrillary acidic protein (GFAP) and the rare cases of oligodendrogliomas reported were GFAP-negative.3,9 The present case is an example of a mixed glioma, with the tumor presenting predominantly astrocytic cells with areas of oligodendroglial differentiation. The decision to classify the present tumor as an astrocytoma was based on the predominance of the astrocytic component and the strong immunoreactivity to GFAP.
Regarding tumor grading, it has been reported that, based on the WHO classification,6 the majority of optic nerve/retinal gliomas in dogs fit into the high-grade astrocytoma category.9 Similarly, the hypercellularity, cellular pleomorphism, relatively high mitotic count (6/10 HPF) and the presence of intratumoral vascular proliferation and necrosis identified on the present tumor led to the classification of a high-grade astrocytoma. Interestingly, despite the high-grade classification of the majority of canine optic nerve/retinal gliomas these tumors rarely metastasize or recur when surgical margins are clean.2,3,9 The most important prognostic feature is the presence of tumor invasion beyond the optic nerve surgical margins, which correlated with tumor recurrence (in the remaining optic pathways) and, in rare cases, tumor extension to the brain.4
Contributing Institution:
Comparative Ocular Pathology Laboratory of Wisconsin - COPLOW
School of Veterinary Medicine
Department of Pathobiological Sciences
University of Wisconsin – Madison
http://www.vetmed.wisc.edu/departments/pathobiological-sciences/
http://uwveterinarycare.wisc.edu/
http://uwveterinarycare.wisc.edu/support-services/laboratory/anatomic-pathology/
JPC Diagnosis: Eye, retina and optic nerve: Astrocytoma, low-grade.
JPC Comment: The moderator, who was also the contributor of this case, has provided an excellent review of this uncommon lesion as well, and this case brings our two-week run of three gliomas(!) to an end.
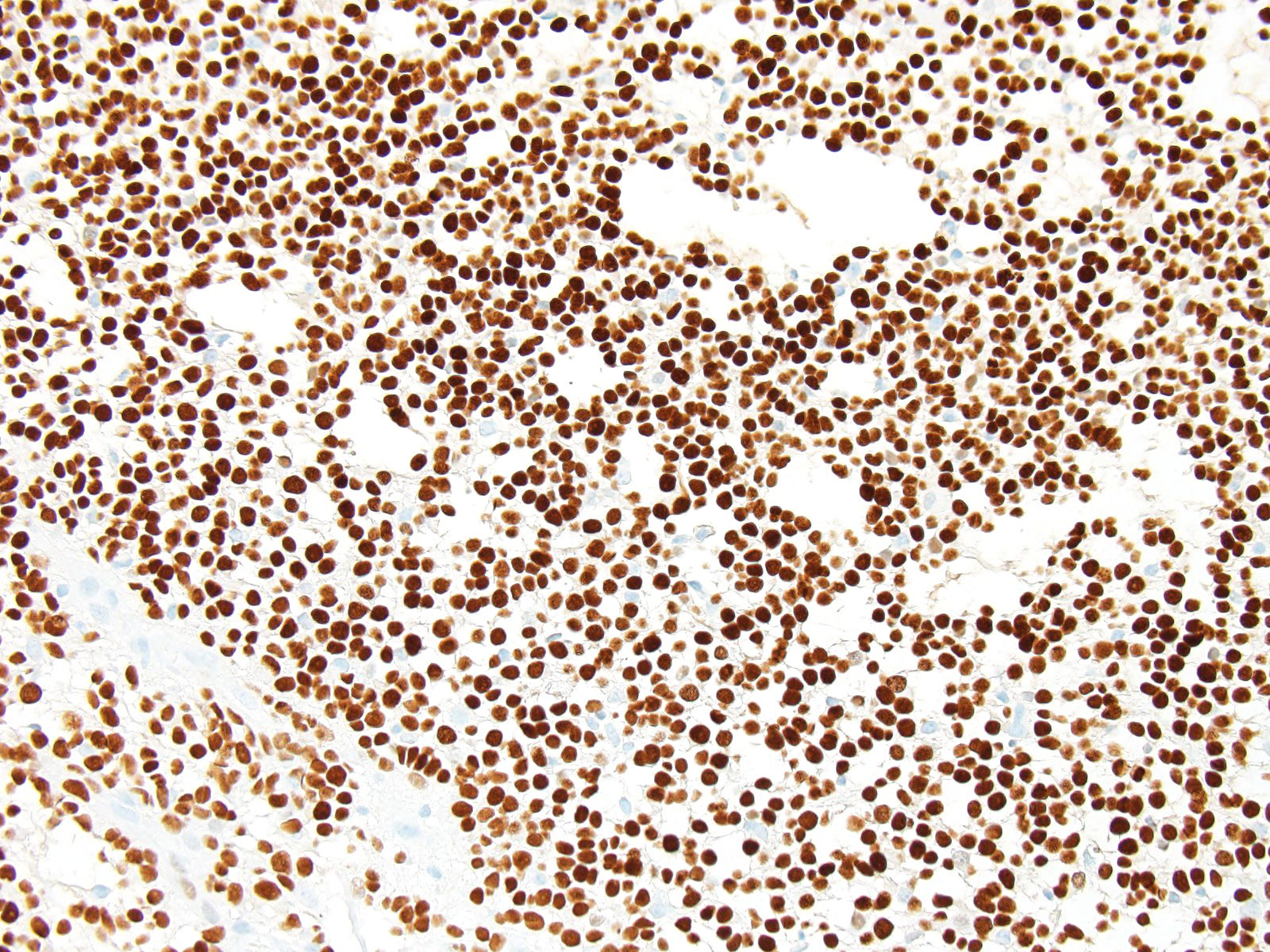
The JPC ran GFAP and Olig-2 on sections from this case and well over 80% of the neoplastic cells were strongly positive for both in this case.
The naming of Olig2, like many other immunohistochemical markers, implies a specificity for oligodendroglia that is unfortunately lacking in the real world of the diagnostic pathologist. Immnohistochemical markers are largely named for their targeted gene product. (Another example of this “false advertising” for immunohistochemical markers is thyroid transcription factor 1, which not only labels both thyroid follicular and C-cells, but also is an excellent marker for pulmonary carcinomas.)
Within the subcutaneous ventricular zone of the developing brain, undifferentiated progenitor cells may ultimately developing to neurons, astrocytes, or oligodendroglia. Expression of Olig2 specifies progenitor cells to pattern glial versus neuronal lineage. By reprocessing the neuronal phenotype in these progenitor cells, only two expression permits production of astrocyte and oligodendrocyte precursors.7 Undifferentiated retinal progenitor cells may also express Olig-2, although oligodendroglia are not found in the retina. In the retina it functions to maintain retinal progenitor cells in an undifferentiated state.8
Olig-2 expression is downregulated in mature astrocytes.7 This may explain the increased expression of Olig 2 in the progression of malignant astrocytomas1, with highest levels in glioblastomas and anaplastic gliomas. A review of labeling indices (LI) in neuroepithelial tumors in humans revealed Olig-2 LI at 43.7% in diffuse astrocytomas, , 59.3 in oligoastrocytomas (mixed gliomas), and 76.1% in oligodendrogliomas.10
From a diagnostic standpoint, Olig2 is obviously widely expressed in both astrocytomas and oligodendrocytomas. It is not expressed in ependymomas, so a negative finding may help to differentiate pilocytic astrocytomas and glioblastomas which morphologically resemble ependymomas from tumors of true ependymal origin.5
While most species do not have oligodendroglia in the retina, dogs and rabbits have a myelinated optic nerve head which and may contain oligodendroglia. However, the strongly positive GFAP in this case rules out the possibility of an oligodendroglioma in the case.
In the conference, the moderator drew the attendees’ attention to the proliferation of blood vessels within the inner layers of the retina (particularly the ganglion cell layer and inner nerve fiber layer) similar to that seen within the tumor as well, suggesting the possibility of local diffusion of growth factors produced by tumor cells. Another incidental change noted by the moderator is the presence of melanin within the connective tissues of the drainage angle, suggestion previous damage to the pigmented epithelium of the ciliary body. While not a problem in this globe at this particular time, infiltration of macrophages may occur at some point in the future to phagocytize the pigment and initiate inflammatory changes that may ultimately result in fibrosis and closure of the drainage angle.
The moderator commented on the thickness of the sclera, which some participants had interpreted as thinned. Three points are helpful in evaluation of scleral thickness – first the moderator says that evaluation of thickness should be made at the limbus. Second, the normal spatial relationship of other structures, such as the iridal leaves will be abnormal in buphthalmic eyes, and finally, comparison to suspected buphthalmic globes to globes from other dogs (even other breeds) may be useful, as the difference in globe size between dogs, even of different breeds, is not very significant.
The JPC diagnosis of astrocytoma, low grade is based on the presence of over 80% of cells which are morphologically identifiable of astrocytes on the HE, a mitotic rate of 2 per 2.37mm2 field, and strong GFAP and Olig2+ positivity (not available to participants before the conference). The moderator said that COPLOW would likely call this a mixed glioma of the retina/optic nerve but the JPC diagnosis was essentially the same.
References:
- Bozinov O, Hohler S, Samans B, Benes L, Miller D, Ritter M, Sure U, Bertalanffy H. Candidate genes for the progression of malignant gliomas identified by microarray analysis. Neurosurg Rev 2008; 31(1):83-90
- Dubielzig RR. The optic nerve. In: Dubielzig RR, Ketring KL, McLellan GJ, Albert DM, ed. Veterinary Ocular Pathology: A Comparative Review. Philadelphia, PA: Saunders Elsevier; 2010:399-417.
- Dubielzig RR. Tumors of the eye. In: Meuten DJ. Tumors in domestic animals. 5ed. Ames, IA: John Wiley and sons Inc.; 2017:892-922.
- Higgins RJ, Bollen AW, Dickinson PJ and Siso-Llonch S. Tumors of the nervous system. In: Meuten DJ. Tumors in domestic animals. 5ed. Ames, IA: John Wiley and sons Inc.; 2017:834-891.
- Ishizawa K, Komori T, Shimada S, Hirose. Olig2 and CD99 are useful negative markers for the diagnosis of brain tumors. Clin Neuropathol, 27: 118-128.
- Koestner A, Bilzer T, Fatzer R. et al. Tumors of neuroepithelial tissue. In: World Health Organization. International Histological Classification of Tumors of Domestic Animals. Histological Classification of the Tumors of the Nervous System of Domestic Animals, 2nd series,Vol. V. Armed Forces Institute of Pathology, Washington, DC,1999; 17–27.
- Marshall CAG, Novitch BG, Goldman JE. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neuroscience 2005; 25(32):7289-7298.
- Nakamura K, Harada C, Namekata K, Harada T. Expression of olig2 in retinal progenitor cells. Develop Neurosci 2006; 17(4):345-349.
- Naranjo C, Schobert C, Dubielzig RR. Canine ocular gliomas: a retrospective study. Vet Ophthalmol. 2008; 11:356-362.
- Suzuki A, Nobusawa S, Natsume A, Suzuki H, Kim Y, Yokoo H, Nagaishi, M, Ikota, H, Nakazawa T, Wakabyashi T, Ohgaki H, Makazato Y. Olig2 labeling index is correlated with histological and molecular classifications in low-grade diffuse gliomas. J Neuro-oncol 2014; 120(2):283-291