Signalment:
Gross Description:
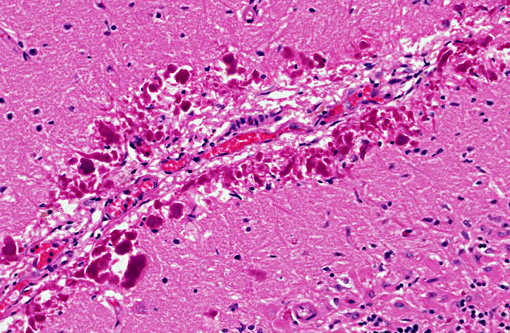
Histopathologic Description:
Rosenthal fibers, particularly at their periphery, show moderate GFAP immunostaining and moderate to strong ubiquitin immunostaining.Â
Morphologic Diagnosis:
Condition:
Contributor Comment:
Alexanders disease in man is a primary astrocytic disorder with approximately 95% of patients harboring mutations in the GFAP gene. The age of onset is variable with cases reported from the prenatal period through until the sixth decade of life. (7) Alexanders disease is classically divided into infantile (0-2 years), juvenile (2-12 years) and adult (>12 years) onset forms however more recently a new classification limited to two categories (type I and type II) has been proposed focusing on lesion distribution and clinical presentation. (10) In the case of the two category classification, system age of onset remained a powerful predictor of disease type. It has been shown that phenotypic pattern and disease course alone more accurately classify cases of Alexander disease and whereas all type I cases presented at an early age, type II cases occurred at all ages although generally with a later onset than type I cases. Type I disease is typically characterized by early onset commonly with seizures, spasticity, or developmental delays and diagnosis can be made based on the presence of at least four of the following five features on MR imaging: extensive cerebral white matter changes with a frontal predominance, a periventricular rim with high signal on T1-weighted images and low signal on T2-weighted images, abnormalities of the basal ganglia and thalami, brain stem abnormalities, and contrast enhancement of the ventricular lining, periventricular tissue or white matter of the frontal lobes, optic chiasm, fornix, basal ganglia, thalamus, dentate nucleus, or brain stem structures.(10,12) Type II disease is typically later in onset and more often presents with signs of hindbrain dysfunction such as ataxia, palatal myoclonus and dysphagia. MR findings in type II disease show hindbrain predominance with atrophy of the medulla oblongata and cervical spinal cord. (4,5,8,9)
There have been seven previous reports of Alexander disease in the dog, all of which predate the new human classification system.(1,2,3,6,11,14) Due to the distribution of lesions in this case with pronounced atrophy of the cervical spinal cord, Type II Alexander disease was diagnosed.
JPC Diagnosis:
Conference Comment:
References:
1. Alema+�-� N, Marcaccini A, Espino L, et al. Rosenthal fiber encephalopathy in a dog resembling Alexander disease in humans. Vet Pathol. 2006; 43: 1025-1028
2. Andrews EJ, Ward BC, Altman NH. Spontaneous animal models of human disease Elsevier; 1980
3. Cox NR, Kwapien RP, Sorjonen DC, et al. Myeloencephalopathy resembling Alexanders disease in a Scottish terrier dog. Acta Neuropathol. 1986; 71: 163-166
4. Farina L, Pareyson D, Minati L, et al. Can MR imaging diagnose adult-onset Alexander disease? AJNR Am J Neuroradiol 2008; 29: 1190-1196
5. Graff-Radford J, Schwartz K, Gavrilova RH, et al. Neuroimaging and clinical features in type II (late-onset) Alexander disease. Neurology 2014; 82: 49-56
6. Ito T, Uchida K, Nakamura M, et al. Fibrinoid leukodystrophy (Alexanders disease-like disorder) in a young adult French bulldog. J Vet Med Sci. 2010; 72: 1387-1390
7. Messing A, Brenner M, Feany MB, et al. Alexander disease. J Neurosci. 2012; 32: 5017-5023
8. Namekawa M, Takiyama Y, Honda J, et al. Adult-onset Alexander disease with typical tadpole brainstem atrophy and unusual bilateral basal ganglia involvement: a case report and review of the literature. BMC neurology 2010; 10: 21
9. Pareyson D, Fancellu R, Mariotti C, et al. Adult-onset Alexander disease: a series of eleven unrelated cases with review of the literature. Brain 2008; 131: 2321-2331
10. Prust M, Wang J, Morizono H, et al. GFAP mutations, age at onset, and clinical subtypes in Alexander disease. Neurology 2011; 77: 1287-1294
11. Richardson JA, Tang K, Burns DK. Myeloencephalopathy with Rosenthal fiber formation in a miniature poodle. Vet Pathol. 1991; 28:536-538
12. van der Knaap MS, Naidu S, Breiter SN, et al. Alexander disease: diagnosis with MR imaging. AJNR Am J Neuroradiolv 2001; 22: 541-552
13. Vandevelde M, Higgins R, Oevermann A. Veterinary Neuropathology: Essentials of Theory and Practice. John Wiley and Sons; 2012
14. Weissenbock H, Obermaier G, Dahme E. Alexanders disease in a Bernese mountain dog. Acta Neuropathol 1996; 91: 200-204
15. Zachary JF. Nervous system. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier Mosby; 2012:776-777.