Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 19
19 February, 2020
Dr.
Molly Church, DVM, PhD, DACVP
Assistant Professor
Pathobiology
University of Pennsylvania School of Veterinary Medicine
Philadelphia, PA
CASE III: KSU VDL (JPC 4032559).
Signalment: 4-month-old female Hereford bovine calf.
History: The calf presented to the referring DVM with opisthotonus, head tilt, and ataxia. The calf was treated with tulathromycin (Draxxin) and dexamethasone for suspected otitis. Amprolium and sucralfate were given for 5 days for suspected nervous coccidiosis. The calf was presented to KSU teaching hospital a month later in sternal recumbency and was unable to stand. The physical examination revealed bilateral dorsolateral strabismus, variable nystagmus, and positive pupillary, menace, and palpebral reflexes with strong tongue tone. The ear exam was normal. There was no response to oxytetracycline, thiamine and dexamethasone. The calf was not eating or drinking and occasionally took few sips of water. The calf was euthanized.
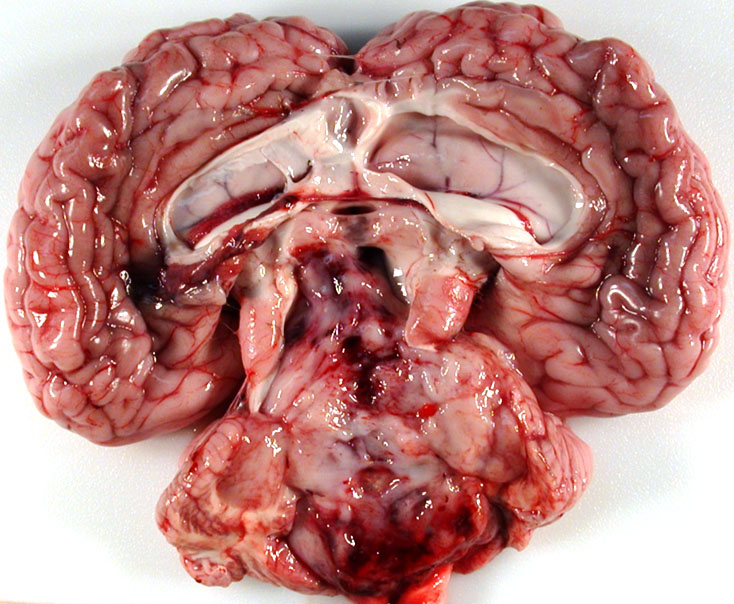
Gross Pathology: The calf was in good body condition. A soft, friable, gelatinous, pale white mass completely filled and expanded the fourth ventricle and compressed the cerebellar vermis and extended cranially and occluded the mesencephalic aqueduct. The lateral ventricles were markedly distended with cerebrospinal fluid that was pale yellow and cloudy. Multifocally, mild to moderate hemorrhages were present within the mass.
Laboratory results: None
Microscopic Description:
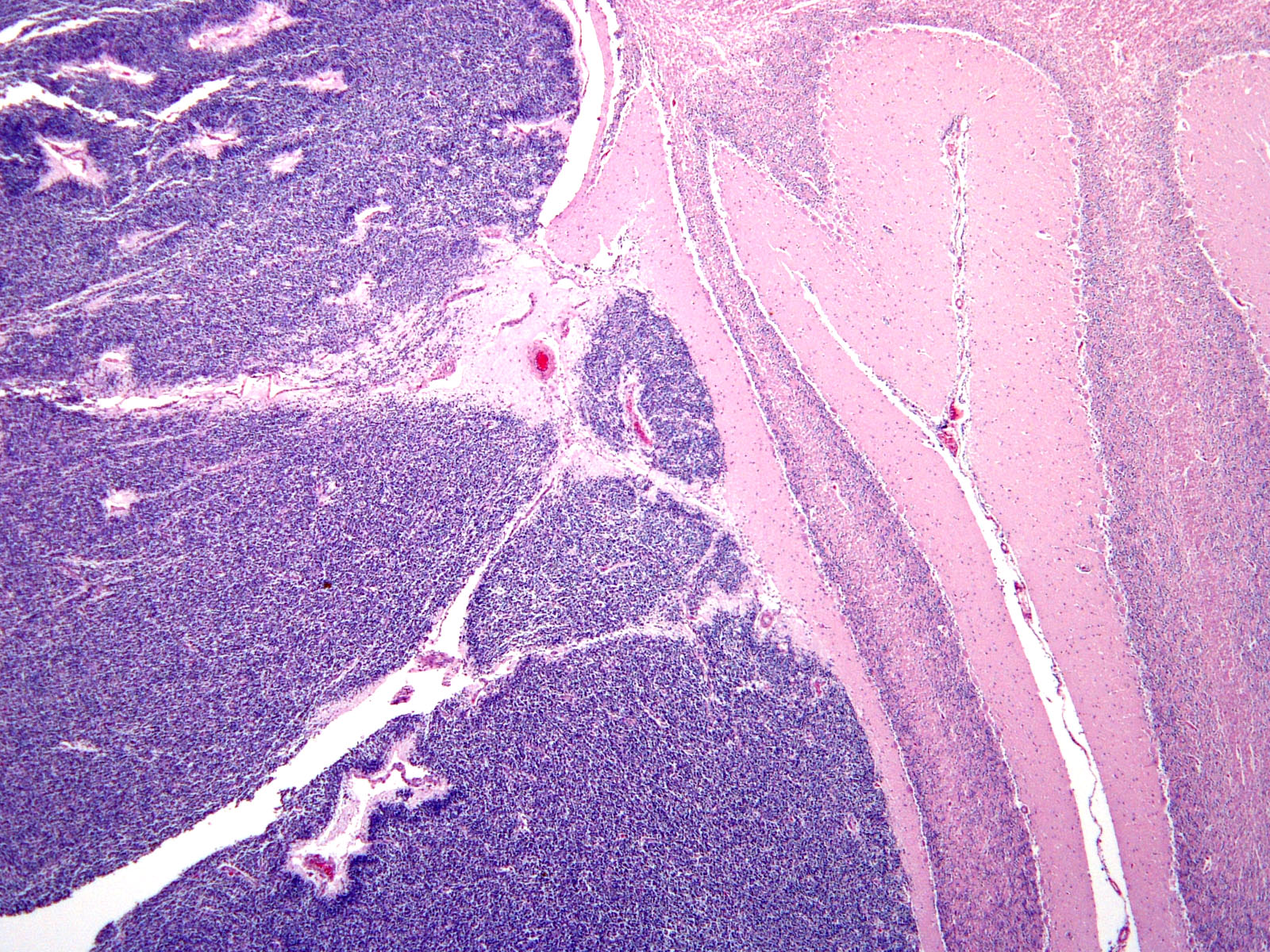
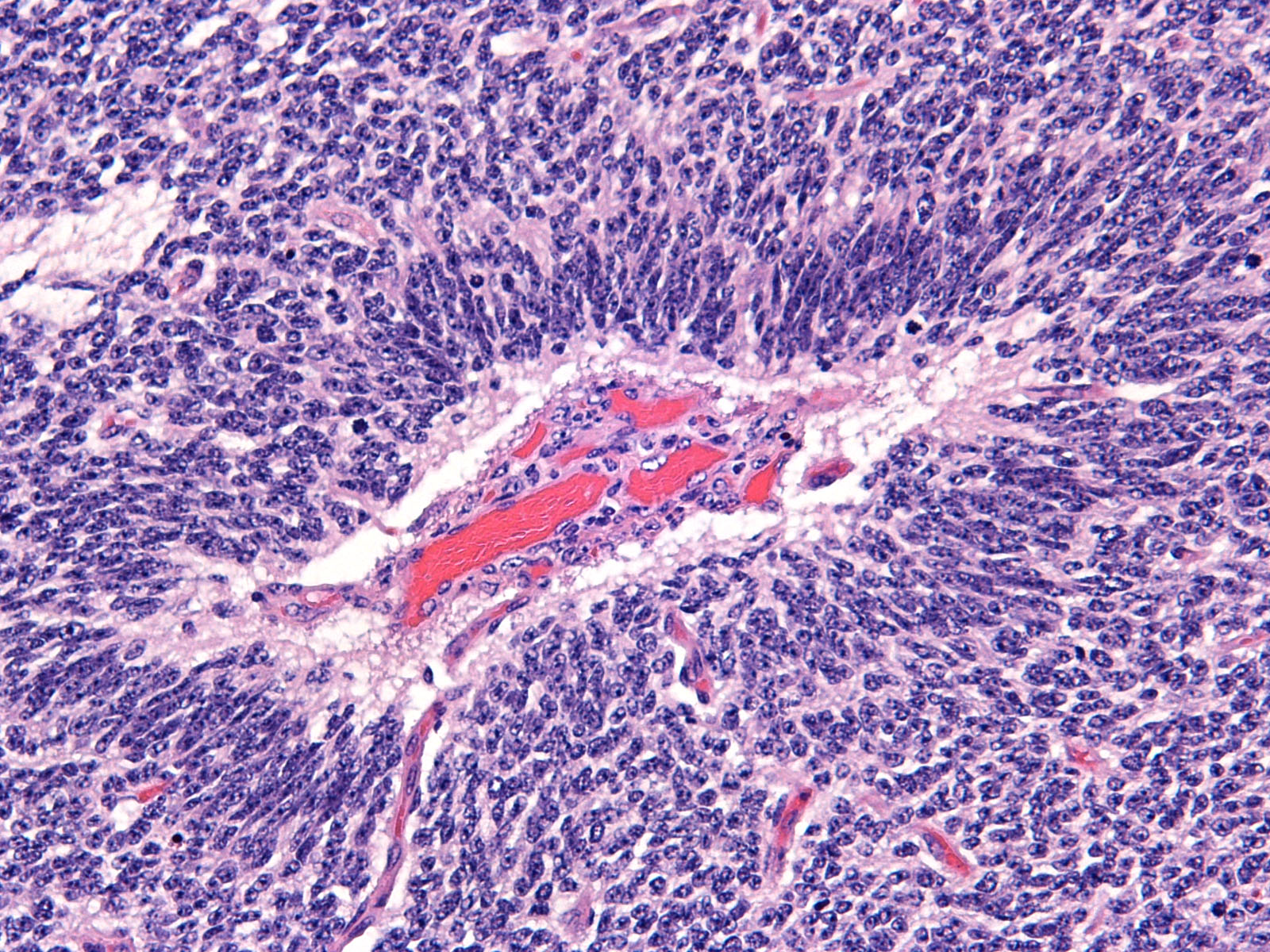
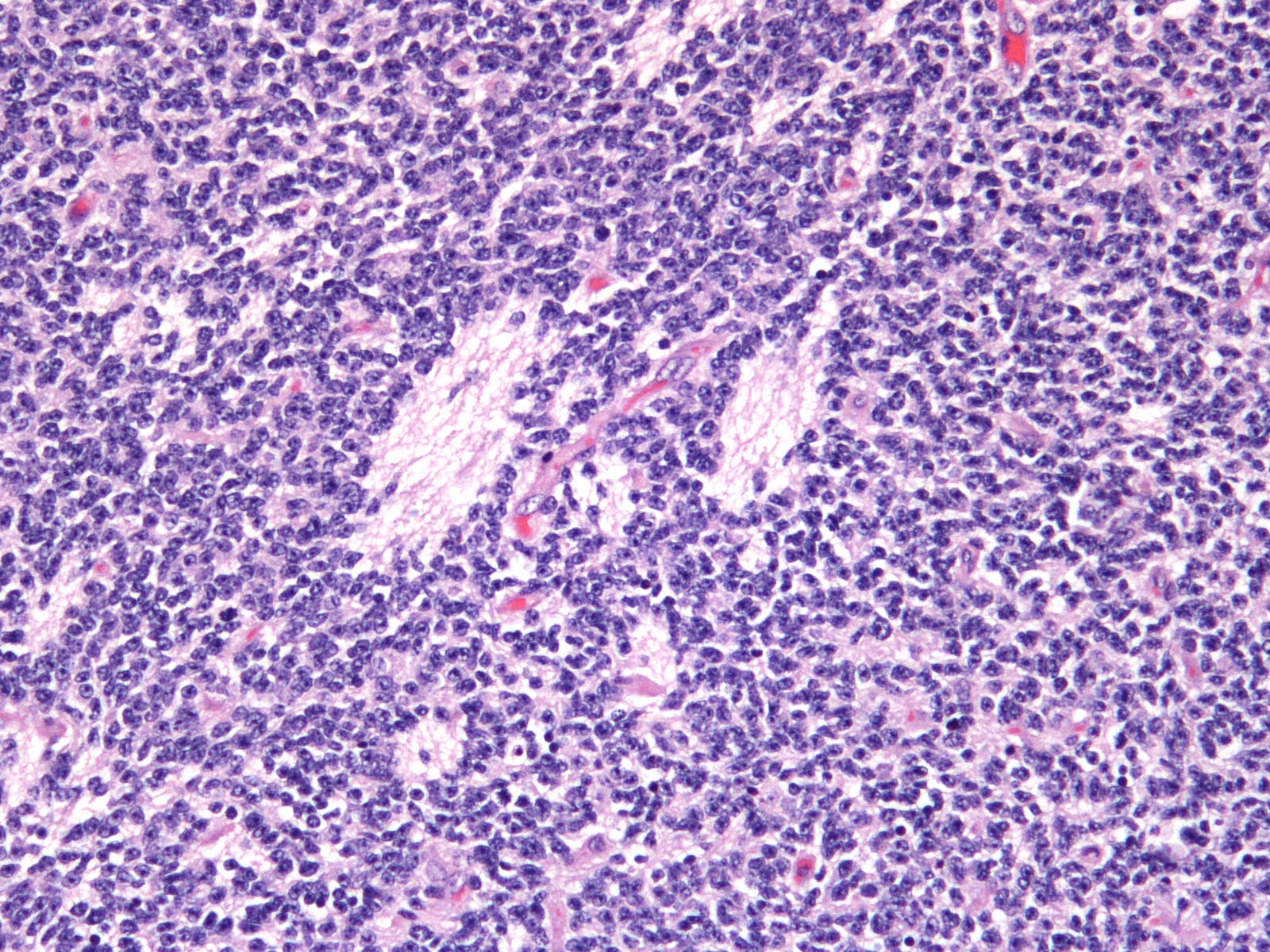
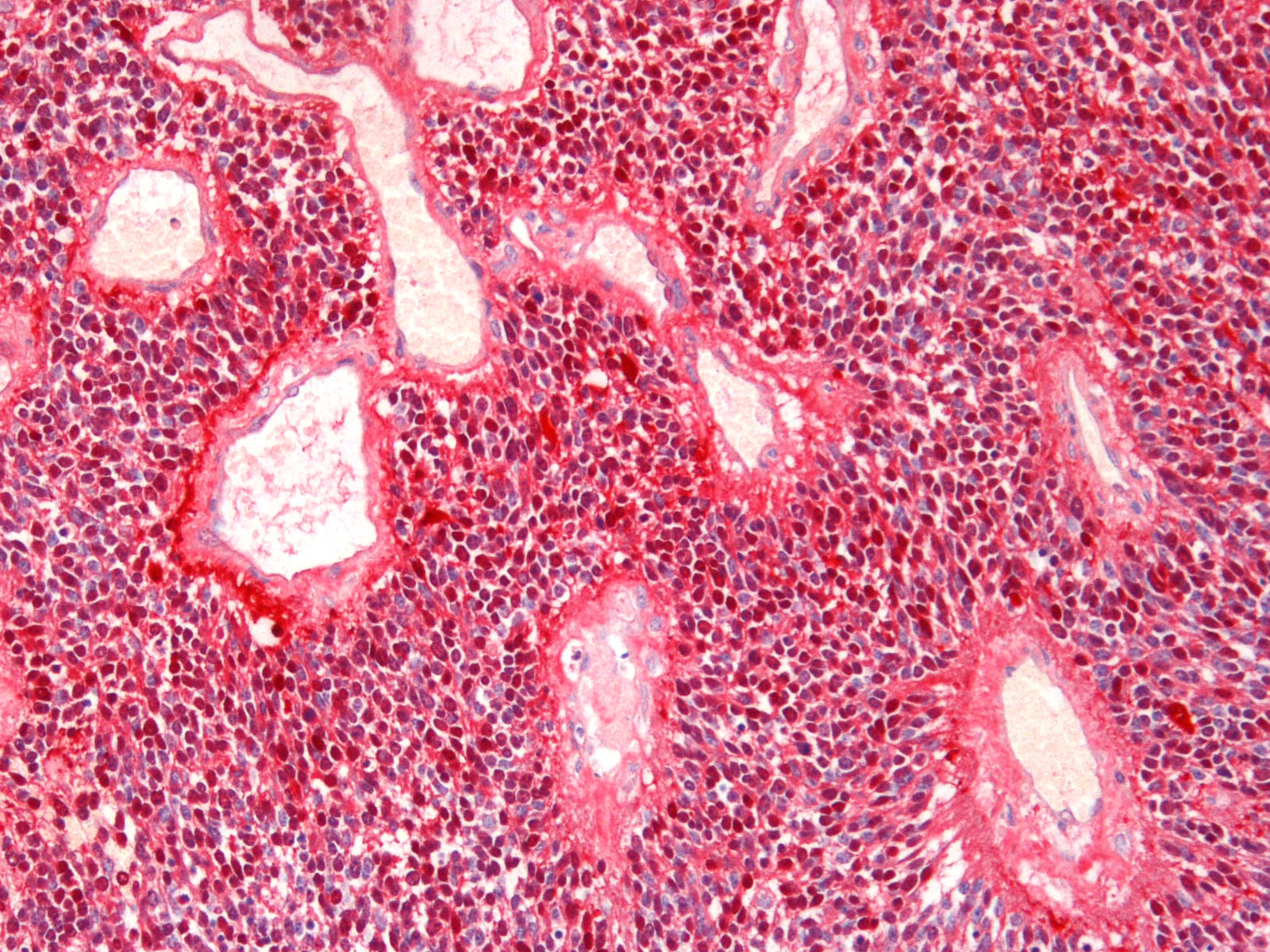
Brain: Expanding the fourth ventricle, compressing and superficially invading the brainstem and cerebellum is a moderately well-delineated, unencapsulated, highly cellular neoplasm composed of round to fusiform cells arranged in sheets supported by a scant fibrovascular stroma. Frequently, three to five cell-layer thick neoplastic cells also palisade around the capillaries (pseudorosettes). Occasionally, the cells palisade around a central fibrillar material (rosettes). The cells have scant eosinophilic cytoplasm and oval to elongate nuclei containing coarsely stippled chromatin and 1-4 nucleoli. There are 10 mitotic figures in ten 400X fields. The cells have variably distinct borders and there is mild anisocytosis and anisokaryosis. Within the neoplasm there are multifocal areas of necrosis, hemorrhage and a few lymphocytic infiltrates.
Histochemical stains are applied to multiple sections of central nervous system tissue (see photomicrographs). The cytoplasmic storage material stains magenta with Periodic acid-Schiff (PAS) and is positive with Luxol fast blue.
Contributor Morphologic Diagnosis:
Brain, cerebellum and brainstem: Medulloblastoma with bilateral marked hydrocephalus of lateral ventricles.
Contributor Comment: Medulloblastoma is a malignant brain tumor that is thought to arise from an undifferentiated germ cells found below the pia mater during fetal and neonatal life.2,11 The terminology medulloblastoma is exclusively used to indicate embryonal tumors arising in the cerebellum while similar neoplasms in other locations of the brain are called primitive neuroectodermal tumors (PNET).2 In animals, it is reported in calves5, young dogs12, cats7, baboons1 and rats9. Medulloblastomas occur more frequently in children (80 %) and is the second most common malignant tumor in the central nervous system of children.5. In children, the neoplasm arises from the vermis and in adults it is located in cerebellar hemisphere. In humans, these neoplasms are seen more commonly in males but no such sex predisposition has been reported in animals.2
Medulloblastomas are malignant tumors that can invade into adjacent neuropil and disseminate via cerebrospinal fluid (CSF). Grossly, medulloblastomas are soft, friable, gray masses that arise from the cerebellum and expand the fourth ventricle. The neoplasm can obstruct the ventricles leading to obstructive hydrocephalus. Microscopically, the cells are round to polygonal, forming sheets or bands with scant cytoplasm and an elongated nucleus sometimes resembling a ?carrot?. Occasionally, the neoplastic cells can form Homer Wright and Flexner-Wintersteiner-like rosettes.2,8
There is variability in the immunohistochemical staining characteristics of cells owing to the stages of differentiation. In one study of canine medulloblastoma, primitive neuroepithelium stained for vimentin and S-100; differentiated neurons stained for neuron specific enolase (NSE) and synaptophysin; differentiated astrocytes labeled positive for glial fibrillary acidic protein (GFAP), vimentin, NSE, and neurofilament.12 In addition, the telomerase activity and c-kit expression were recently reported in canine medulloblastomas.9
Immunohistochemistry was performed in the current case for NSE, synaptophysin, GFAP, vimentin, cytokeratin, and S-100. The neoplastic cells were immunopositive for vimentin and S-100 and negative for GFAP, NSE, synaptophysin and cytokeratin, suggesting that the neoplastic cells are undifferentiated. Differentiation along neuronal and glial lineage is less common in animals than in humans.2
Contributing Institution:
Department of Diagnostic Medicine and Pathobiology
Kansas State Veterinary College of Veterinary Medicine
1800 Denison Avenue
Manhattan, KS 66506
http://www.vet.k-state.edu/depts/dmp/index.htm
JPC Diagnosis: Cerebellum: Primitive neuroectodermal tumor (medulloblastoma).
JPC Comment: Medulloblastoma is a form of primitive neuroepithelial tumor that likely arises from the external germinal cell zone, which lies directly beneath the meninges in the developing cerebellum.6 Normally, during fetal development, cerebellar granular cells develop in the external germinal zone then migrate past Purkinje cells to form the granule cell layer.
Activation of the hedgehog pathway has been shown not only to regulate normal growth, but is also involved in the tumorigenesis of several neoplasms, including medulloblastomas.4 More specifically, the Patched gene (Ptc) controls growth and pattern formation in early neural development and the adult cerebellum.3 Ptc gene encodes a Sonic hedgehog (Shh) receptor and a tumor suppressor protein. While many subtle aspects of signaling have not been completely elucidated, it is clear that a complex interaction between Shh and Ptc is required for normal development. Shh binds to Ptc, activates smoothened (Smo) which leads to over expression of Gli-1 and some Wnt and TGF-ß gene families.3,4 (Hedgehog effectors Gli-1 and BclII have also been shown to be overexpressed in medulloblastomas).3 Ptc-knockout mice (which allow for unregulated expression of Shh target genes have a high incidence of PNET formation ? eight percent of Ptc-heterozyogous mice develop tumors as early as 5 weeks.6 Tumor incidence increases with age in Ptc-heterozygous mice with an incidence of about 30% at six months of age.6 In humans, Ptc mutation has been associated with basal cell carcinoma, fibroma, medulloblastoma and rhabdomyosarcoma.4
References:
1. Berthe J, Barneon G, Richer G, Mazue G. Medulloblastoma in a baboon (Paio paio). Lab Anim Sci. 1980; 30: 703-705.
2. Cummings J, de Lahunta A. Veterinary Neuropathology, Mosby, St. Louis, MO, 1995; 378-379.
3. Goodrich LV, Milenković L, Higgins KM, Scott MP: Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277:1109-1113, 1997
4. Hahn H, Wojnowski L, Specht K, Kappler R, Calzada-Wack J, Potter D, Zimmer A, Müller U, Samson E, Quintanilla-Martinez L, Zimmer A: Patched target Igf2 is indispensable for the formation of medulloblastoma and rhabdomyosarcoma. J Biol Chem 275:28341-28344, 2000
5. Jolly RD, Alley MR. Medulloblastoma in calves: A report of three cases. Vet Pathol. 1969; 6: 463-468.
6. Kim JYH, Nelson AL, Algon SA, Graves O, Sturla LM, Goumnerova LC, Rowitch DH, Segal RA, Pomeroy SL: Medulloblastoma tumorigenesis diverges from cerebellar granule cell differentiation in patched heterozygous mice. Developmental Biology 263:50-66, 2003
7. Kitagawa H, Koie, Kanayama K, Sakail T. Medulloblastoma in a cat: clinical and MRI findings. J Small Anim Pract. 2003; 44: 139-142
8. Koestner A, Bilzer T, Schulman F, Summers B, Van Winkle T: Histological classification of tumors of the nervous system of domestic animals. In: World Health Organization, International Histological Classification of Tumors of Domestic Animals, ed. Armed Institute of Pathology, Washington, DC, 1999; 25-25
9. Krinke G, Kuafmann W, Mahrous A, Schaetti, P. Morphologic characterization of spontaneous nervous system tumors in mice and rats. Toxicol Pathol. 2000; 28: 178-192
10. Madrioli L, Biserni R, Panarese S, Morini M, Gandini G, Bettini G. Immunohistochemical profiling and telomerase activity of a canine medulloblastoma. Vet Pathol. 2011; 48: 814-816
11. Maxie M G, Youssef S: The nervous system. In: Jubb, Kennedy, and Palmer?s Pathology of domestic animals. Vol. 1. 5th ed., Elsevier, Edinburg, 2007; 450-451
12. Steinberg H, Galbreath EJ. Cerebellar medulloblastoma with multiple differentiations in a dog. Vet Pathol. 2005; 35:543-546.