WSC 2023-2024, Conference 19, Case 4
Signalment:
1-year-old male Visayan hornbill (Penelopides panini)
History:
The animal was found dead from suspected hypothermia following an enclosure heating malfunction. The animal was submitted for necropsy. No other birds were affected.
Gross Pathology:
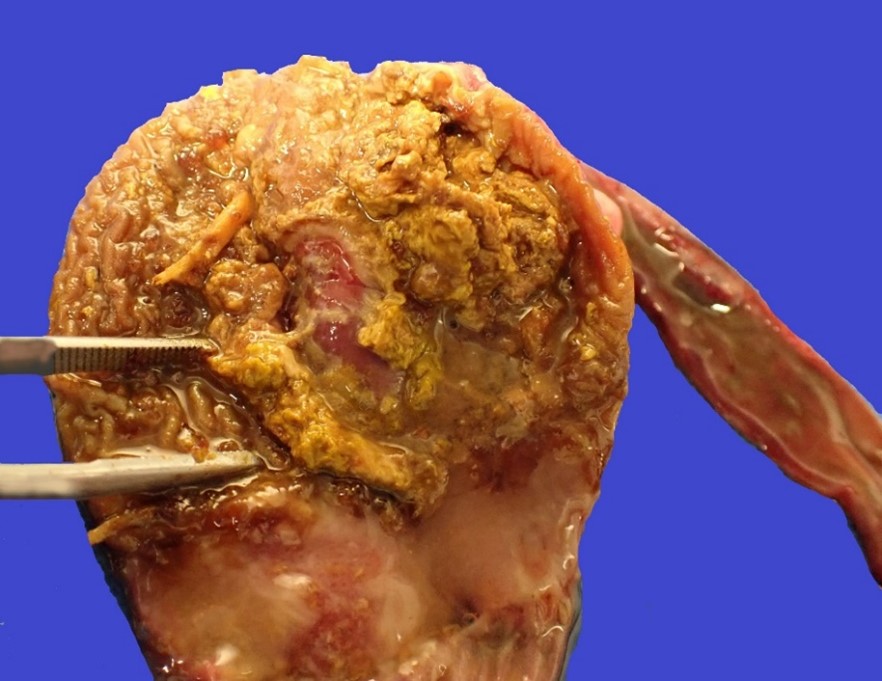
The bird weighed 0.5 kg (slightly underweight) and was in lean body condition with ample skeletal muscle bulk and minimal body fat. The ventriculus contained a moderate amount of yellow, soft, diphtheritic material replacing the koilin layer centered on a central, ovoid, 14 x 18 mm mucosal ulcer. The adjacent duodenum was diffusely and transmurally reddened and contained moderate volumes of red-tinged mucoid fluid overlying eroded intestinal mucosa. Concurrent macroscopic findings included moderate diffuse splenomegaly and marked diffuse pulmonary edema.
Laboratory Results:
Microbiology: Anaerobic cultures of the ventriculus and small intestine isolated a moderate growth of Clostridium perfringens.
Aerobic cultures produced mixed scant growth of Proteus spp., Escherichia coli, and Enterococcus spp. Selective cultures for Salmonella, Campylobacter, Shigella, and Yersinia were sterile.
PCR: The bacterial isolate was identified as Clostridium perfringens by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) analysis.
A PCR toxin profile identified alpha toxin; beta, epsilon, iota, beta-2, and enterotoxin were all absent.
Microscopic Description:
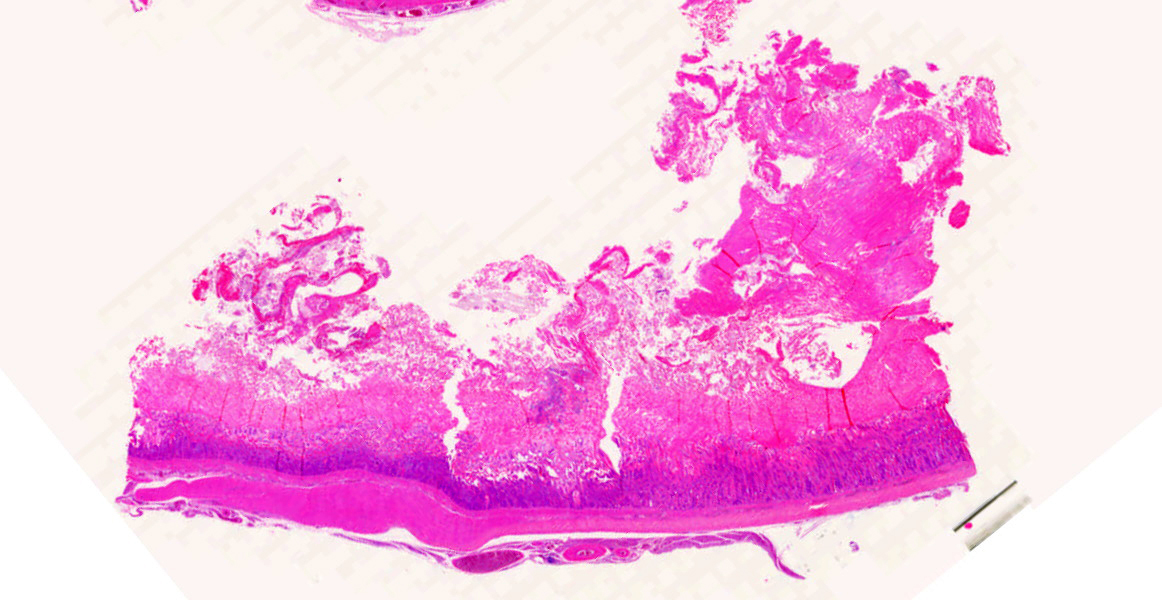
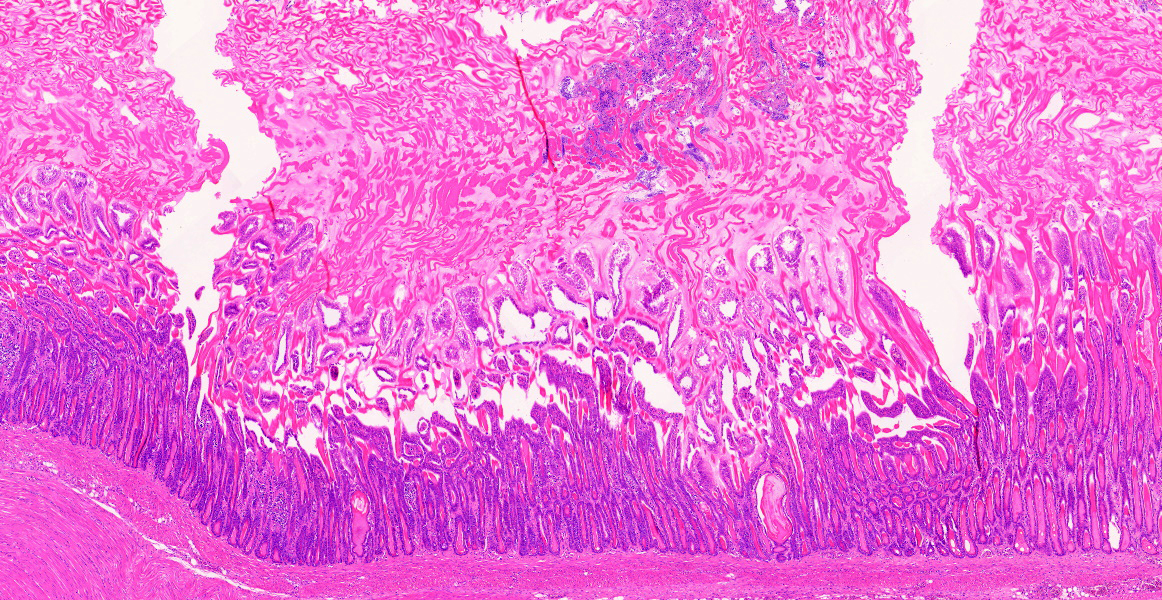
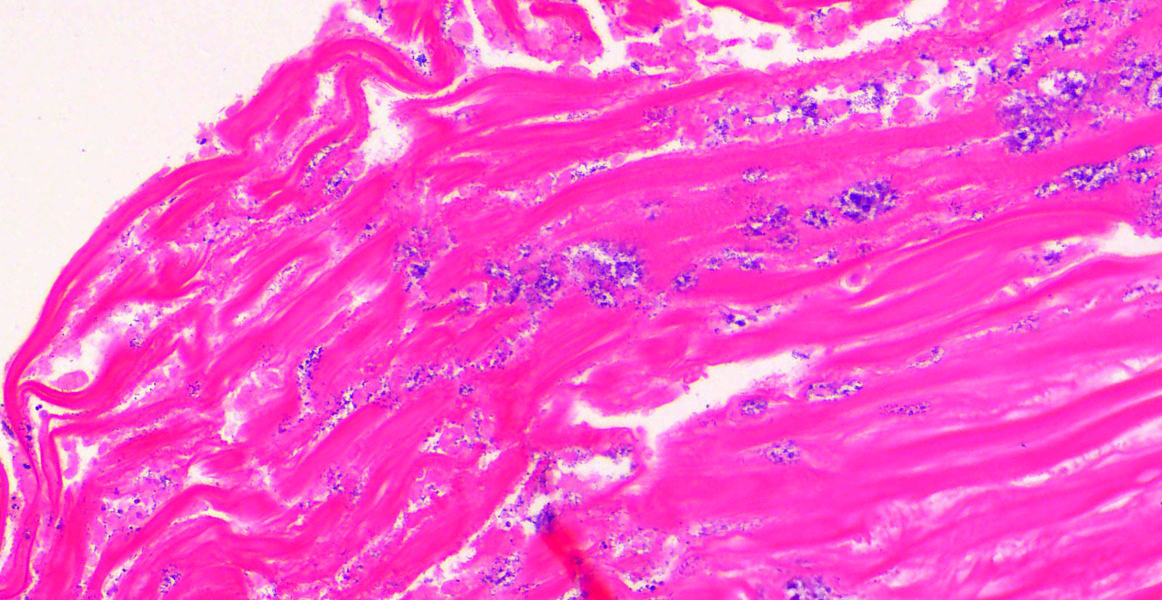
Ventriculus-duodenal junction: Overall affecting approximately 70-80% of the examined ventriculus, there is widespread multifocal-to-coalescing erosion and degeneration of the koilin layer, characterized by superficial erosive scalloping and variable separation of the superficial horizontal koilin matrix layers due to fibrillary to finely globular protein degeneration with numerous intralesional small interspersing colonies of gram-positive rod-like bacteria that range in size from 1-2 x 3-6 µm. Multifocal areas of more extensive koilin necrosis are seen, characterised by complete loss of horizontal koilin matrix architecture and replacement by hypereosinophilic amorphous koilin protein debris. Where extensive, koilin necrosis is confluent with regions of ventricular mucosal ulceration, with tissue replaced by hypereosinophilic lytic cytonuclear debris admixed with abundant fibrin, moderate heterophilic and histiocytic inflammation, and numerous small (approx. 50-micron diameter) to medium-sized (approx. 400-micron diameter) colonies of dense rod-like bacteria, together forming a fibrinonecrotising (diphtheritic) membrane. Remnant epithelial mucosa exhibits variable features of crypt dilatation (containing either fine fibrillary hypoeosinophilic proteinaceous content or hypereosinophilic koilin matrix) and epithelial cell degeneration (including cell swelling, loss of apical cilia, loss of cytoplasmic detail, karyorrhexis and nuclear pyknosis), the latter multifocally leading to lytic necrosis (cytoplasmic rupture, exudation of degenerate cytoplasm and karyolitic nuclei). Mild to moderate mixed inflammatory infiltrates (mostly degenerate heterophils, lymphocytes, and plasma cells with occasional macrophages) are seen throughout the lamina propria. Ventricular smooth muscle layers are multifocally disrupted by infiltrating heterophils with variable degrees of myofibrillar swelling, fiber splitting, striation loss, and nuclear pyknosis and karyorrhexis. Mild heterophilic infiltrates occasionally infiltrate through the subserosal layers, and are mostly associated with variably degenerate adipose tissue. Myenteric plexi are unaffected. Ventricular blood vessels exhibit relative increases in circulating heterophils (granulocytosis). Small sections of duodenum are also present in the examined section, which exhibit mild to moderate superficial autolytic mucosal exfoliation. Where better preserved, mild multifocal areas of lytic mucosal necrosis are observed with associated loss of villi and crypts, plus blunting and fusion of remaining adjacent villi. Prominent lymphoplasmacytic infiltrates are seen within the lamina propria, with occasional heterophils scattered throughout. The submucosa, muscle layers, and serosa appear generally unremarkable. Gram stain confirms the prescence of numerous gram-positive, rod-like bacteria, consistent with Clostridium perfringens. Ziehl-Neelsen stain for acid-fast organisms is negative. PAS stains were negative for fungal organisms.
Pancreas: Histologically unremarkable.
Contributor’s Morphologic Diagnosis:
Severe, subacute, multifocal-to-coalescing, fibrinonecrotising and ulcerative ventriculitis with koilin necrosis and intralesional gram-positive rod-like bacteria consistent with Clostridium perfringens, gizzard.
Contributor’s Comment:
The gram-positive anaerobic spore-forming bacteria Clostridium perfringens is a major enteric pathogen in animals, represented by 7 distinct toxinotypes (A to G) based on the detection of 6 toxin genes: alpha (cpa gene), beta (cpb), epsilon (etx), iota (itx), enterotoxin (cpe), and necrotic enteric B-like toxin (netb).1,2 Whilst typically found in intestinal flora of healthy birds, physiologic stress may promote C. perfringens-associated necrotic enteritis (NE), a significant disease caused by types A (cpa only), C (cpa and cpb), and G (cpa and netb-toxin).3,5 Necropsy findings in this case include distended and friable small intestines with multifocal-to-coalescing fibrinonecrotising mucosal ulcers covered by “Turkish-towel”-like diphtheritic pseudomembranes.2 C. perfringens may subsequently invade the portal system and biliary tract, causing cholangiohepatitis.2 Histologically, gram-positive rod-like bacteria may be seen initially adhered to villous tips and later embedded within foci of mucosal to transmural intestinal necrosis.2
Outbreaks in avian collections occur infrequently, with clinical infection characterized by sudden onset of high mortality and small intestinal mucosal necrosis. NE typically affects domestic poultry but has also been described in more wild and exotic avian species such as lorikeets, macaws, toucans, and crows.1-3,5,9 C. perfringens can be ubiquitous in the environment, particularly if soil-based substrate is used in the enclosure, therefore review of enclosure hygiene protocols is usually recommended in zoological collections when cases occur.
This case was particularly unusual in that the ventriculus was the primary organ affected, rather than the small intestine, which is more typical in other avian species. The most immediate cause of death was attributed to bacteraemic sepsis, based on the observation of heterophilic infiltrates within other viscera (i.e., liver, lung, and spleen) plus necrotizing hepatitis associated with similar intralesional gram-positive rod-like bacteria. To the contributor’s knowledge, this is the first reported case of C. perfringens-associated ventriculitis in a hornbill, and in the Order Bucerotiformes.
Contributing Institution:
International Zoo Veterinary Group
Station House, Parkwood Street
Keighley, West Yorkshire
United Kingdom
http://www.izvg.co.uk/pathology/pabout.htm
JPC Diagnoses:
1. Ventriculus: Koilin erosion and ulceration, multifocal, subacute, moderate, with glandular hyperplasia and numerous extracellular bacteria.
2. Ventriculus: Vasculitis, fibrinoid and heterophilic, multifocal, moderate.
3. Adipose: Atrophy, diffuse, moderate.
JPC Comment:
Clostridium perfringens and its various subtypes and toxins are well-known to veterinary students, residents, and practitioners, who have developed all manner of mnemonics dedicated to classifying C. perfringens types based on toxin production. This pathogen is so well-known because it is so ubiquitous in the environment and is the cause of many distinct enteric diseases in humans and in a wide variety of animal species, particularly among herbivores.8
The currently classification scheme classifies C. perfringens types based on the production of particular “typing toxins,” but all isolates produce numerous other toxins and hydrolytic enzymes that contribute to virulence.8 In addition, genes encoding beta, epsilon, and iota toxins are encoded on plasmids which also contain conjugation loci, and transfer of epsilon toxin plasmids from type D strains to type A strains, with subsequent production of epsilon toxin, which effectively turns type A into type D, has been observed, meaning that the beloved classification scheme is essentially based on the plasmid complement carried by an individual organism.8
The only clostridial toxin isolated in this case, alpha toxin (CPA), is a chromosomally-encoded toxin that is produced by all strains of C. perfringens.8,10 CPA is a phospholipase that compromises cellular function by degrading phosphatidylcholine and sphingomyelin, both of which are components of eukaryotic cell membranes.10 Release of CPA causes damage to erythrocyte and other cell membranes, resulting in cell lysis via phospholipid degradation and the activation of other cellular mechanisms that culminate in lysis. CPA also activates the arachidonic acid metabolic pathways, resulting in the production of thromboxanes, leukotrienes, and prostaglandins, resulting in inflammation and vasoconstriction.10 In small amounts, CPA impaires leukocyte migration and causes their intravascular aggregation at the periphery of lesions, leading to a reduction in blood flow, subsequent hypoxia and necrosis, and the creation of an anaerobic niche for the proliferation of C. perfrigens.10
The role of CPA in mammalian intestinal disease is controversial. C. perfringens type A causes yellow lamb disease, a rare form of enterotoxemia in lambs that is characterized by depression, anemia, icterus and hemoglobinuria.10 It is generally assumed that these clinical signs and the associated gross and microscopic lesions are caused by the action of CPA, but no definitive proof is available.10 Similarly, C. perfrigens type A is blamed for enteritis, abomasitis, and enterotoxemia in cattle, horses, goats, and pigs, though no definitive evidence for CPA’s role in the pathogenesis of these diseases has been discovered.10 The picture is further muddied by the detection of CPA in the intestinal contents of clinically healthy animals, making the presence of CPA diagnostically irrelevant for type A diseases in many species.10 C. perfringens type A is known to produce gas gangrene (also known as malignant edema) in many domestic animals and in humans and CPA has long been assumed to be the causative agent; however, in a recurring theme, there is no concrete evidence that the disease produced by the bacterium is the result of CPA.10
The controversy surrounding CPA extends to avian diseases. While Clostridium perfrigens type A is frequently found as the sole agent in a variety of enteric avian diseases, it is unclear if CPA is responsible for the observed lesions.3,5,8 In fact, CPA was long considered the key virulence factor for necrotizing enteritis in broiler chickens until recent research demonstrated that NetB, not CPA, is the main lesion-causing virulence factor produced by the newly-described C. perfringens type G strain.5,7
This case prompted robust discussion from conference participants, who noted, as did the contributor, how the ventriculus is an unusual and surprising location for C. perfringens-associated disease. Participants were surprised at the extent of the koilin destruction and ulceration, which would be an unusual manifestation of clostridial disease and is more intense than would be expected from stress induced from a relatively short period of cold exposure.
In general, koilin erosion and ulceration in the ventriculus of birds has many associated conditions, and is likely a multifactorial disease. In chickens, koilin erosion and ulceration is considered a syndrome and termed “gizzard erosion and ulceration,” or GEU. Numerous causes for GEU have been proposed, including starvation, genetic factors, inappropriate feed, toxins, nutritional deficiencies, and infections. C. perfringens is one infection agent that has been putatively associated with this condition and may have caused the koilin erosion and ulceration in this case.4
Also of particular interest was the multifocal heterophilic vasculitis present through the ventriculus. The cause of this vasculitis is not apparent in the examined section and vasculitis is not a lesion commonly associated with clostridial infection in birds, leading participants to believe that this likely represents a separate pathologic process. As heterophilic vasculitis and koilin ulceration can be caused by fungal infections in birds,6 participants scrutinized the section for fungal hyphae, but no fungal elements were observed. Other possible causes of vasculitis include bacterial sepsis, viral disease, and immune-mediated vasculitis. In this case, the cause of the vasculitis is ultimately unknown.
Participants also noted fat atrophy in the examined section, corresponding to the clinical history that the bird was underweight. The fat atrophy, vasculitis, and severe koilin layer ulceration and erosion comprise an unusual constellation of lesions which prompted an array of hypothetical pathogeneses, none of which could be definitely confirmed by the examined section alone. In the end, participants chose to separate each lesion into separate morphs due to the uncertain relationships between them.
References:
- Asaoka Y, Yanai T, Hirayama H, et al. Fatal necrotic enteritis associated with Clostridium perfringens in wild crows (Corvus macrorhynchos). Avian Pathol. 2004;33 (1):19-24.
- Boulianne M. Clostridial Diseases. In: Swayne DE, Boulianne M, Logue CM, et al., eds. Diseases of Poultry. 14th ed. Wiley-Blackwell; 2020.
- De Santi M, Schocken-Iturrino RP, Casagrande MF, et al. Necrotic enteritis caused by Clostridium perfringens in blue and gold macaws (Ara ararauna). J Avian Med Surg. 2020;34(1):65-69.
- Gjerve AG, Kaldhusdal M, Eriksen GS. Gizzard erosion and ulceration syndrome in chickens and turkeys: a review of causal or predisposing factors. Avian Pathol. 2013;42(4):297-303.
- Grau-Roma L, Navarro M, Blatter S, et al. Clostridium perfringens-associated necrotic enteritis-like disease in coconut lorikeets (Trichoglossus haematodus). Vet Pathol. 2021;58(2):423-427.
- Jeffrey JS, Chin RP, Shivaprasad HL, Meteyer CU, Droual R. Proventriculitis and ventriculitis associated with zygomycosis in ostrich chicks. Avian Dis. 1994;38(3):630-4.
- Lee KW, Lillehoj HS. Role of Clostridium perfringens necrotic enteritis B-like toxin in disease pathogenesis. Vaccines (Basel). 2022;10(1):61.
- Rood JI, Adams V, Lacey J, et al. Expansion of Clostridium perfringens toxin-based typic scheme. Anaerobe. 2018;53:5-10.
- Trupkiewicz J, Garner MM, Juan-Salles C. Passeriformes, Caprimulgiformes, Coraciiformes, Picipformes, Bucerotiformes and Podiformes. In: Terio K, Mcaloose D, St. Leger J, eds. Pathology of Wildlife and Zoo Animals, 1st ed. Academic Press;2018.
- Uzal FA, Vidal JE, McClane BA, Gurjar AA. Clostridium perfringens toxins involved in mammalian veterinary disease. Open Toxinology J. 2010;2:24-42.