Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 9
1 November 2018
CASE I: 13-12484 (JPC 4050022-00).
Signalment: Two day old male warmblood horse, Equus caballus
History: This colt presented for bloody diarrhea, lethargy, and anorexia for ~1 day. His birth was not witnessed and he was noticed to be standing and nursing although the mare’s udder was tight and he was not nursing well. He passed tan diarrhea at that time. Later that morning the diarrhea became bloody and he was increasingly lethargic. At presentation to Oregon State University Lois Bates Acheson Veterinary Teaching Hospital he was lateral, dull, ~ 5-10% dehydrated, and minimally responsive. Mucous membranes were pale, tacky, cold, and injected with a prolonged CRT. There were petechiae in the oral mucous membranes and in the pinna. Preliminary diagnosis was sepsis and despite supportive care there was little improvement. Due to the guarded prognosis the foal was euthanized and a necropsy was performed.
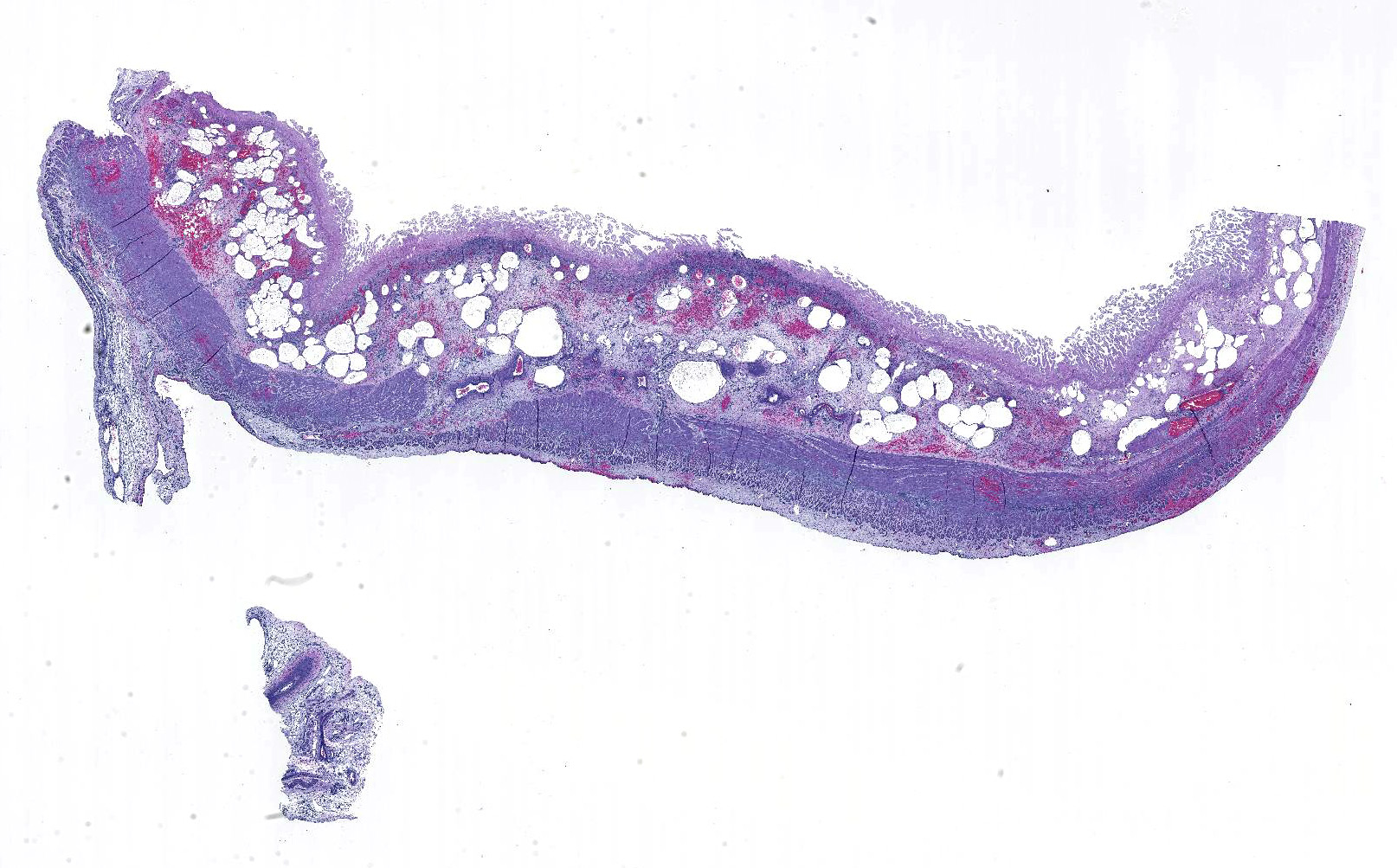
Gross Pathology: Jejunal content was hemorrhagic, with multifocal zones of mucosal necrosis up to 50 x 15 cm throughout. There were multiple gastric ulcers with hemorrhagic gastric content and bloody liquid material throughout the small and large intestine. Petechiae and ecchymoses were present in the endocardium and kidneys.
Laboratory results: CBC: Leukopenia (2810; RI: 6000-120000/ul), HCT 48.7% RI: 32-48%), hypoproteinemia (5.4; RI: 6.0-8.5 g/dL), hyperfibrinogenemia (400; RI: 100-400 mg/dl), lymphopenia (1208; RI: 1500-5000/ul), moderate neutropenia with a left shift (674; RI: 3000-6000/ul; bands 141 RI: 0-100ul).
Complete Chemistry: Mild azotemia (BUN 30; RI: 8-23 mg/dl, Crea 2.3; RI: 0.9-1.7 mg/dl), mild hypoglycemia (Glu 51; RI: 79-109), mild hyperbilirubinemia (3.4 RI: 0.8-2.6 mg/dl), moderate to marked hyperphosphatemia (10.1; RI: 1.9-4.1 mg/dl), increased SDH (12.9; RI: 2.4-7.2 U/L
IgG > 800 mg/dl
Blood culture: Actinobacillus equuli two colony types
Fecal c. difficile enterotoxin ELISA: positive
Fecal c. perfringens enterotoxin ELISA: negative
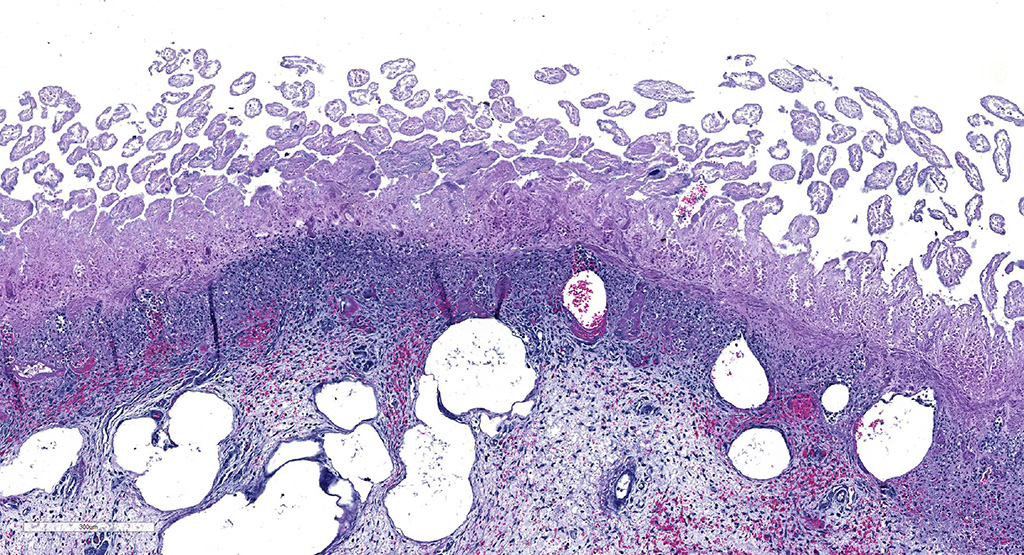
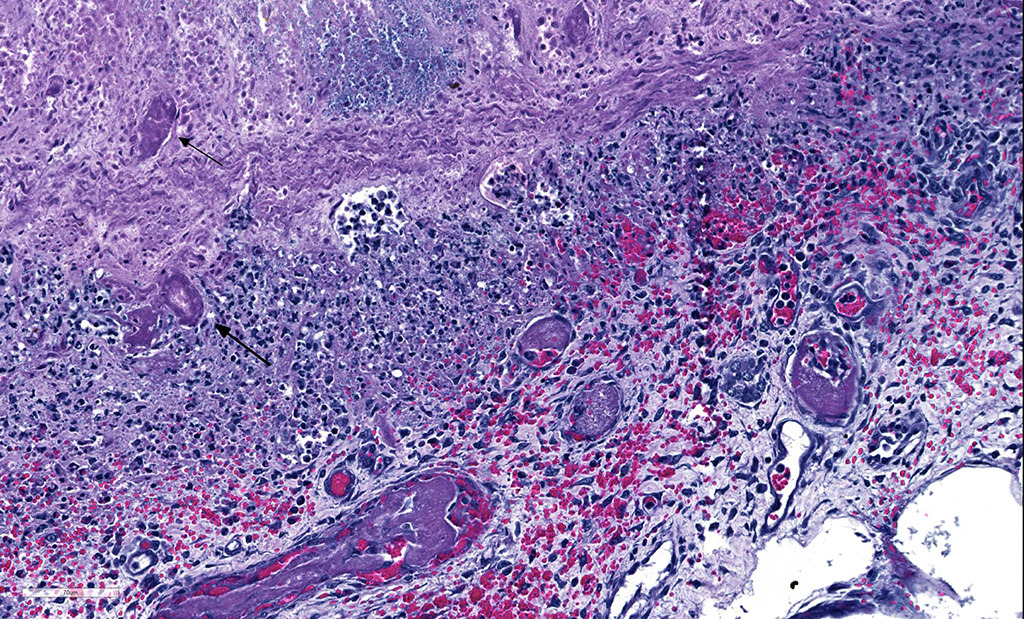
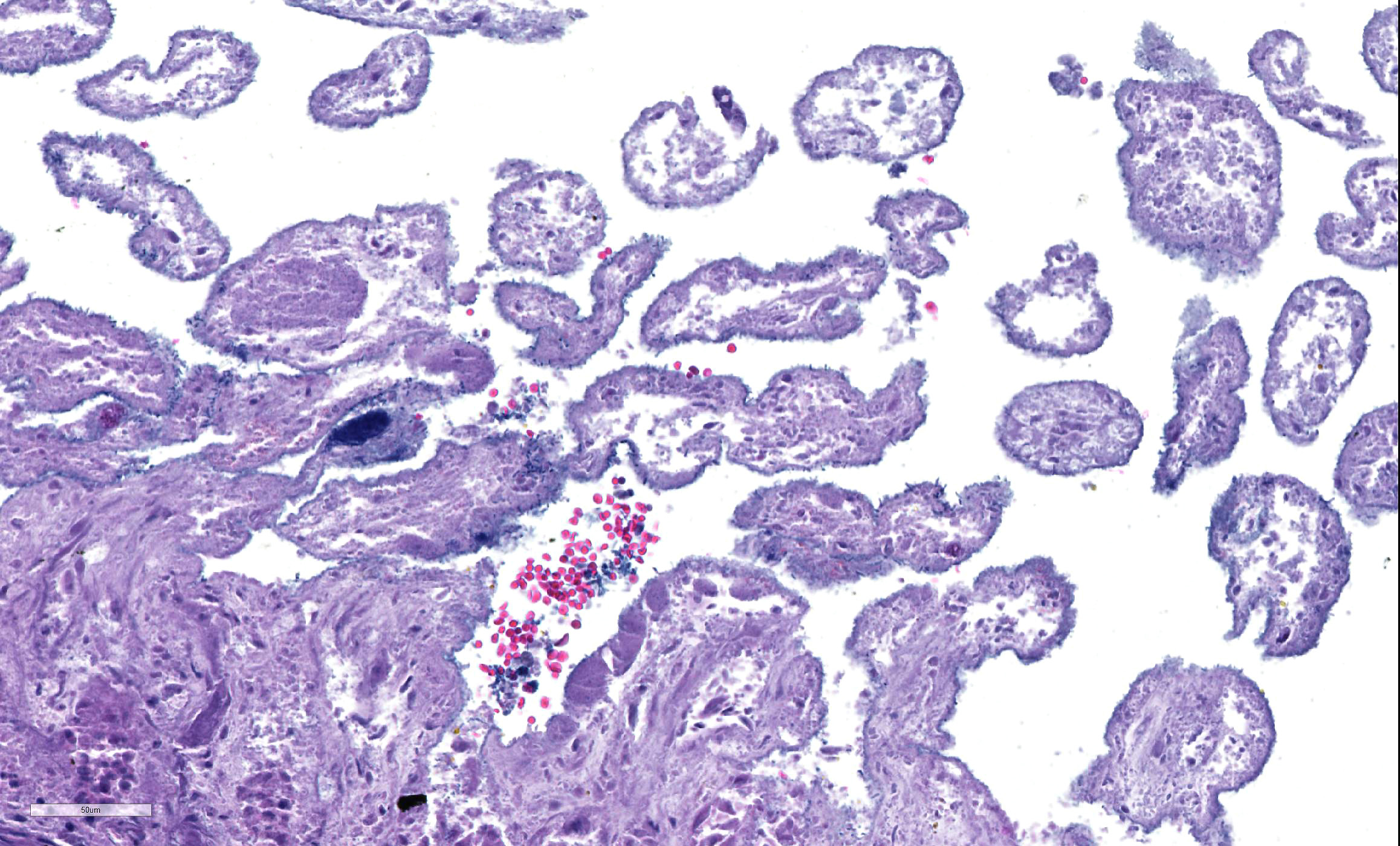
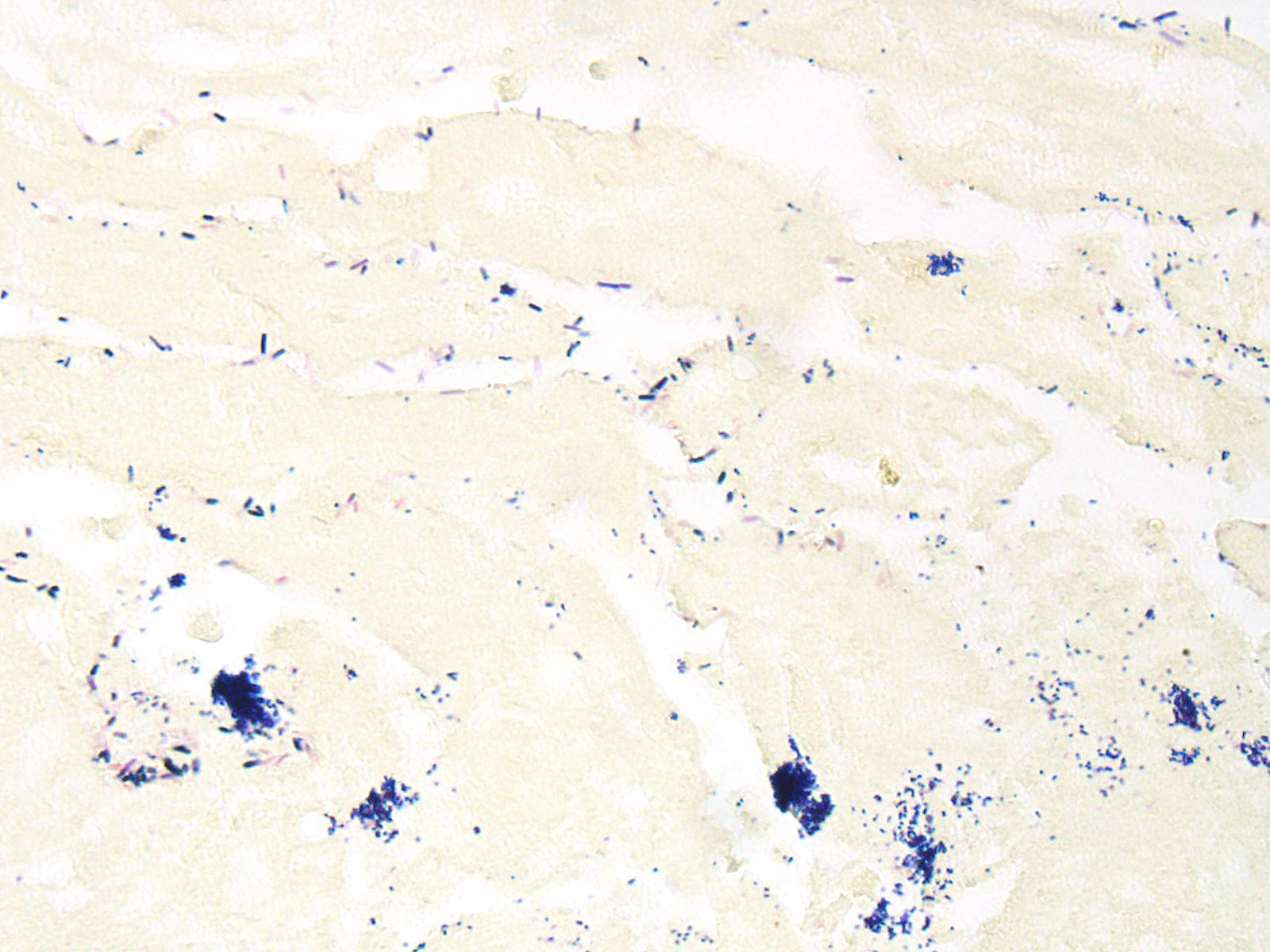
Microscopic Description: Small intestine: There are multifocal extensive areas of transmural hemorrhage. The submucosa is markedly edematous and there are multifocal fibrin thrombi with overlying mucosal necrosis. Within areas of necrosis there are numerous vessels occluded with amorphous, smudgy, eosinophilic deposition (fibrin thrombi) in the lamina propria and the tips of the villi are hypereosinophilic with necrosis and cellular debris. Also within affected areas there are multiple colonies of bacilli. The tissue is inflamed with mixed degenerate inflammatory cells and the base of the mucosa is expanded by plump fibroblasts (proliferative fibroplasia) and numerous small vessels lined by plump endothelium (reactive vasculature).
Contributor’s Morphologic Diagnoses:
Small intestine: Severe multifocal transmural necrohemorrhagic enteritis with fibrin thrombi and bacilli consistent with clostridia
Contributor’s Comment: Clostridium difficile is a gram-positive, anaerobic, spore-forming bacillus associated with diarrhea and (entero)colitis in humans and other mammals3. Colitis is most common in most species but the small intestine is affected in rabbits and foals3. In horses, Clostridium difficile, has been incriminated in hemorrhagic enteritis in foals and colitis in all ages and foals may be affected within the first few days of life5. The disease causes severe acute-to-peracute enterocolitis likely due to dysbacteriosis with clostridial overgrowth secondary to antibiotic therapy, stress, or dietary changes3. Although prior antibiotic treatment and/or hospitalization are significant predisposing factors in horses that develop C.difficile-associated disease (CDAD), disease may occur without prior treatment or hospitalization1,2. Foals < 7 days old without prior antibiotic therapy or hospitalization may frequently develop CDAD2. The primary virulence factors of C. difficile are toxin A (TcdA) and toxin B (TcdB) which act synergistically3. TcdA causes widespread damage to the mucosa (enterotoxic) allowing TcdB to affect epithelial cells (cytotoxic)3. TcdA enters the epithelial cell through endocytosis by coated pits and escapes into the cytoplasm by acidification of the endolysosome. In the cytoplasm, toxin inactivates Rho and other GTPase necessary for the regulation of cytosolic actin filaments leading to a loss of cell-to-cell contacts, increased paracellular permeability, and ultimately cell death3. TcdA and TcdB also initiate the inflammatory cascade including generation of reactive oxygen intermediates and elaboration of IL-8 and macrophage inhibitory protein 2 (MIP-2), both of which cause influx of neutrophils leading to host tissue damage3. TcdA also causes monocyte production of IL-1, IL-6, IL-8, and TNFα, and macrophage expression of COX-2 leading to release of prostaglandin E2 which inhibits sodium chloride and water absorption in the intestine and enterocyte chloride secretion3. Clostridium perfringens is also an important cause of enteric disease but usually causes disease in more proximal segments of the gastrointestinal tract3. In foals, enteritis with extensive necrosis can occur in the small intestine due to both C. difficile and C. perfringens3. In this case the ELISA for C. perfringens toxin was negative.
This case demonstrates classic gross and histologic lesions of CDAD. Additional lesions, petechiation and ecchymosis, have been described in horses with CDAD and have been attributed to endotoxic shock and/or disseminated intravascular coagulation1. The most common histologic findings in foals with confirmed C. difficile infection are necrotizing or necrohemorrhagic enteritis and submucosal thrombosis,1 which were features of this case. Other infectious differentials for this type of enteritis in horses includes C. perfringens type C enterotoxemia, salmonellosis, or ehrlichial enteritis1. Culture and toxin assay should be interpreted together because culture sensitivity is below 100% and C. difficile can be isolated from a small number of healthy horses1.
Actinobacillus equuli was isolated from the blood culture. A. equuli is a common cause of neonatal septicemia and death, with disease course ranging from abortion to early neonatal death to survival for several days with development of microabsesses in many organs5. A. equuli can occasionally be found as an opportunist in pathological tissue5. In this case there were fibrin thrombi identified in the kidney and edema of the choroid plexus that may have been caused by septicemia by A. equuli. The lesions in the intestine were most significant and the positive ELISA for C. difficile confirmed the diagnosis of CDAD. Mucosal damage due to Clostridium infection with subsequent A. equuli entry and sepsis is a likely sequence of events and has been suggested in a report of a foal with multiple concurrent diseases including CDAD and A. equuli4.
Contributing Institution:
Oregon State University College of Veterinary Medicine
Department of Biomedical Sciences and Veterinary Diagnostic Laboratory
http://vetmed.oregonstate.edu/diagnostic
JPC Diagnosis: Small intestine: Enteritis, necrotizing, diffuse, severe, with submucosal hemorrhage, edema and emphysema and numerous adherent robust bacilli.
JPC Comment: The contributor provides an excellent review of the subcellular events associated with C. difficile infection in foals. In this species, both foals and horses are equally susceptible to infection, with antibiotic therapy and hospitalization being the main predisposing factors for the development of this disease in adult horses.2
difficile has been the cause of disease in a wide variety of mammalian species. First identified from feces of clinically healthy human babies in the 1930s, the organism was originally named Bacillus difficilis because of the difficulties encountered in cultivating it. In humans, most infected people will remain symptomatic, with the remainder developing variable GI signs ranging from watery diarrhea to pseudomembranous colitis.2 As in horses, hospitalization and antibiotic administration is a predisposing factor. Most C. difficile-positive infants, however, lack clinical signs, possibly due to lacking receptors for the toxin.
In humans, C. difficile associated disease (CDAD) was always assumed to affect individuals of any age, except during the neonatal period as it was thought that this specific group may lack specific C. difficile toxin receptors. Although between 25 and 70% of human neonates are colonized with C. difficile, these microorganisms have been largely considered part of the commensal microbiota. Recently, however, two 9 or 18 month-old children were diagnosed with CDAD, providing evidence that C. difficile is a potential cause of bloody diarrhea in neonates and young infants. In most animal species, CDAD is not age-dependent. The exception to this are pigs, which are almost exclusively affected during the neonatal period, up to approximately one week of age.6
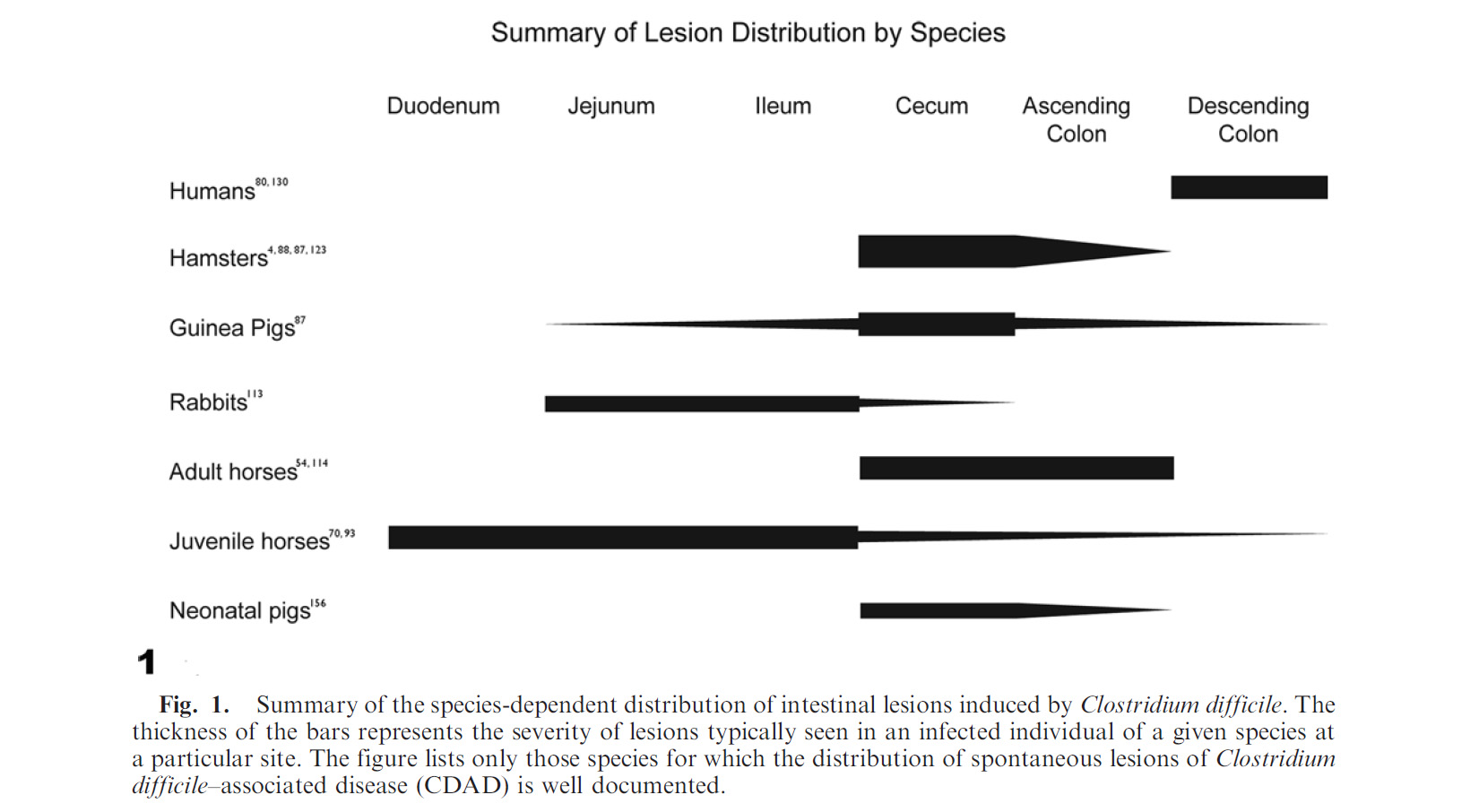
C. difficile-associated disease (CDAD) affects a wide range of other mammals – while always resulting in enterocolitis, its manifestation varies widely with the affected species. Table 1 summarizes the severity of disease in various species by enteron segment.
Table 1. Summary of lesion distribution by species.3
In rodents, CDAD is primarily cecal, resulting in ulcerative and rarely proliferative typhlitis and death. In pigs, the disease results in ulcerative typhitis or colitis with development of “volcano ulcers”. An additional gross finding of mesocolonic edema (as well as diarrhea) makes CDAD a differential diagnosis for edema disease in swine.2 The difference is that C. difficle infections occurs in young piglets (1-7 days of age), while edema disease is a disease of weanling age pigs. In rabbits, the lesion is primarily seen in the small intestine and concentrated in the ileum, often following antibiotic administration. It has also been reported sporadically in dogs, cats, ostriches, prairie dogs, and experimentally in non-human primates.3
Diagnosis of CDAD is usually based on a combination of clinical history, often with previous antibiotic administration or hospitalization, characteristic microscopic lesions in appropriate segments of the enteron, and most importantly, ELISA testing of feces and/or intestinal for C. difficile-specific TcdA of TcdB toxins. Culture is useful because the carrier rate in horses is low, but care should be taken as: a) a few healthy horses (usually up to 5% although there is great variation in the literature) can be carriers of C. difficile, and b) occasionally non-toxigenic strains may be present in the intestine of horses, so any isolate should ideally be typed by PCR to confirm the presence of the genes encoding the major toxins of this microorganism. The latter is not routinely done in most veterinary diagnostic laboratories. Gross lesions, and to a lesser extent microscopic lesions are non-specific as to etiology, and in the horse, may resemble those of other diseases such as C. perfringens type C, salmonellosis, Potomac horse fever, and NSAID toxicity.2
In reviewing the slide, the moderator commented on the presence of numerous bacilli lining the necrotic villi as a relatively non-specific finding that should NOT be interpreted as evidence of clostridial infection, unless an appropriate immunohistochemical stain discloses that they are all of the same etiology. Many bacilli (and not just clostridia) will adhere to denuded mucosa. In addition, The moderator also pointed out that the presence of fibrin thrombi in the submucosa as evidence that the changes in the mucosa are truly antemortem and not simple autolysis.
References:
- 1 Diab SS, Rodriguez-Bertos A, Uzal FA: Pathology and diagnostic criteria of Clostridium difficile enteric infection in horses. Vet Pathol 2013:50(6):1028-1036.
- 2 Diab SS, Songer G, Uzal FA: Clostridium difficile infection in horses: a review. Vet Microbiol 2013:167(1-2):42-49.
- 3 Keel MK, Songer JG: The comparative pathology of Clostridium difficile-associated disease. Vet Pathol 2006:43(3):225-240.
- 4 Lohr CV, Polster U, Kuhnert P, Karger A, Rurangirwa FR, Teifke JP: Mesenteric lymphangitis and sepsis due to RTX toxin-producing Actinobacillus spp in 2 foals with hypothyroidism-dysmaturity syndrome. Vet Pathol 2012:49(4):592-601.
- 5 Maxie MG: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals 5th ed. Philadelphia: Elsevier Limited; 2007.
- Uzal FA, Navarro MA, Li J, Freedman JC, Shrestha A, McCline BA. Comparative pathogenesis of enteric clostridial infections in humans and animals. Anaerobe 2018; 53:11-20.