Signalment:
On day two post challenge, this vaccinated chicken was found to be quiet. It was lethargic and anorexic on day three post inoculation and dead on day four. Virus was isolated at day two, three and four days post inoculation from both upper respiratory and cloacal swabs. Post mortem examination was performed and organs submitted for histopathological examination.
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
The HA gene is the primary determinant of high pathogenicity in chickens, but a proper constellation of all eight gene segments is required for the maximal expression of virulence potential. The cleavage of the HA into the HA1 and HA2 proteins is essential for the virus to be infectious and produce multiple replication cycles. In moderately pathogenic AI viruses, trypsin-like proteases found in restricted anatomical sites, such as respiratory and intestinal epithelial cells or within secretions of the respiratory lumen, are required to cleave the HA and thereby produce infectious virus. However, in HPAI viruses the HA is cleaved by ubiquitous furin proteases present in many cells of numerous visceral organs, the nervous system and the cardiovascular system. Trypsin-like proteases will also cleave the HA of HPAI viruses.
An issue separate from HA cleavability is receptor binding between the receptor-binding site of the HA and the receptor on the host cells. This is a poorly understood phenomenon but impacts both host specificity (hostadaptation) and cell or tissue tropism within the host. This may restrict virus replication to specific cells, tissues and organs. Changes in the receptor-binding site of the HA have been shown to change the host range of an influenza virus. Both the virus and the host impact receptor binding.
Avian influenza viruses exert pathological effects on avian cells by two mechanisms: necrosis and apoptosis. Necrosis has been associated with intense virus replication and demonstration of abundant AI viral nucleoprotein in the nucleus and cytoplasm. Apoptotic cell death has been demonstrated in various cell culture systems. In chicken embryos, apoptosis and necrosis may share similar biochemical features and their differentiation is neither always easy nor clear.
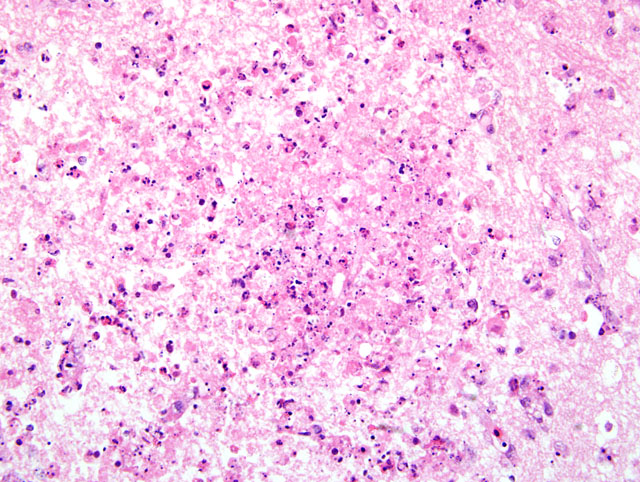
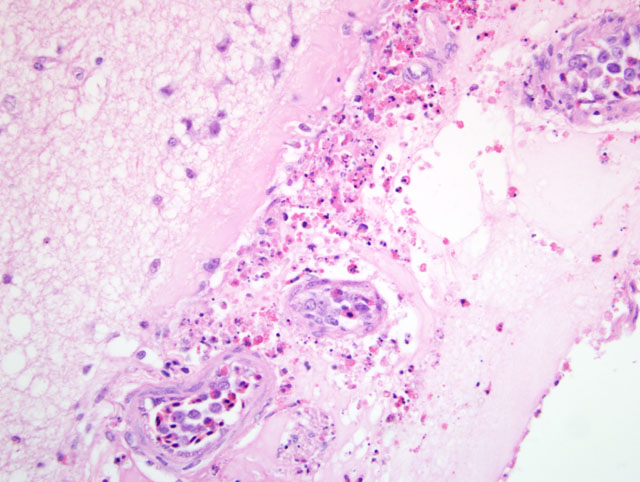
Typically, histological lesions consist of multi-organ necrosis and/or inflammation. The most consistently and severely affected tissues are the brain, heart, lung, pancreas and primary and secondary lymphoid organs. Lymphocytic meningoencephalitis with focal gliosis, neuronal necrosis and neuronophagia are common, but edema and hemorrhage may be seen. Lesions in the brain have abundant associated influenza virus proteins in neurons.(6)
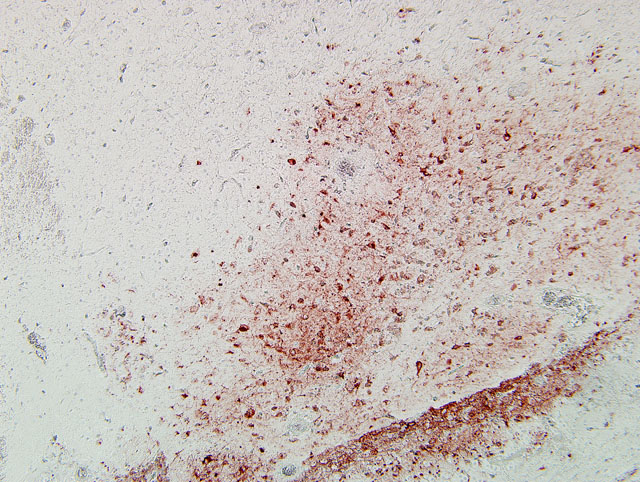
In our case, immunoperoxidase staining revealed antigen in a variety of cells in the brain including endothelial cells, neurons, perivascular cuffs, ependymal cells as well as inflammatory cells in the meninges. Lesions were present in most organs including the lung, heart, kidney, spleen, ventriculus, small and large intestines, and pancreas. In most cases involving chickens, HPAI is fulminating with some birds being found dead prior to observance of any clinical signs. If the disease is less fulminating and birds survive for 3-7 days, as in our case, individual birds may exhibit nervous disorders such as tremors of the head and neck, inability to stand, torticollis, opisthotonos and other unusual positions of head and appendages. Respiratory signs are less prominent than with less pathogenic AI viruses, but can include rales, sneezing and coughing.
The virus is transmitted by direct contact between infected and susceptible birds or indirect contact through aerosol droplets or exposure to virus-contaminated fomites. Aerosol generation from the respiratory tract is a significant mode of transmission because of high virus concentrations in the respiratory tract, but the large volume of lower concentration AI virus in infected feces makes fomites a major mode of transport.(6)
The importance of the human health implications of AI infections were revealed during the 1997 Hong Kong outbreak, in which the H5N1 virus was shown to have infected 18 people, six of whom died.(1) This virus, which is panzootic in poultry, continues to spread and pose a major challenge to animal and human health. While the H5N1 virus transmits zoonotically from infected poultry to humans, often with fatal consequences, such transmission remains inefficient. Although the virus replicates efficiently in diseased humans, it has not yet adapted to efficient human-to-human transmission. The H5N1 virus therefore continues to challenge our understanding of interspecies transmission of influenza viruses.(4)
JPC Diagnosis:
Conference Comment:
Vitamin E deficiency affects chicks, causing encephalomalacia, exudative diathesis, and nutritional myopathy. Encephalomalacia usually manifests clinically between days 15 and 30 of life as ataxia or paresis, and grossly, hemorrhages are often noted on the cerebellar surface. Microscopic lesions include ischemic necrosis, demyelination, and neuronal degeneration, most prominently in the cerebellum, striatal hemispheres, medulla oblongata, and mesencephalon.(3) The submitted case is characterized by more severe inflammation than would be expected in nutritional encephalomalacia.
Fungal encephalitis of young chickens, turkey poults, and quail chicks is caused by the dematiaceous thermophilic fungus Ochroconis gallopavum (Dactylaria constricta var. gallopava). Gross lesions may involve both the cerebellum and cerebrum, and range from hard, gray, well-circumscribed foci to focally extensive areas of red discoloration. Histologic lesions include infiltration by numerous heterophils, macrophages, and multinucleated giant cells centering on areas of necrosis with numerous readily-apparent dematiaceous fungal hyphae, which are conspicuously absent in this case.(2)
The contributor provides a succinct review of AI in general, and H5N1 HPAI specifically, which since 2003 has produced natural infection in a number of species, including humans, domestic pigs, dogs, cats, tigers, leopards, stone martins, and civets. Migratory aquatic birds, particularly those belonging to the orders Anseriformes and Charadriiformes, are the reservoirs of all AI viruses, and typically do not develop disease due to infection. In poultry, as described by the contributor, clinical signs and pathology depend not only on the pathotype of AI virus (i.e. low pathogenicity [LP] or high pathogenicity [HP]), but also on host species, age, sex, immune status and environmental factors, among others. The gross and histologic lesions of HPAI listed by the contributor are nonspecific, and the differential diagnosis includes velogenic Newcastle disease virus, septicemic fowl cholera (Pasteurella multocida), heat exhaustion, water deprivation, and several toxins. For LPAI, which affects the respiratory tract and causes reduced egg production, the differential diagnosis is long, and includes lentogenic Newcastle disease virus, avian pneumovirus and other paramyxoviruses, infectious laryngotracheitis (gallid herpesvirus 1), infectious bronchitis (avian coronavirus), chlamydiosis and mycoplasmosis, among others. As such, the diagnosis of AI infection requires the employment of virologic and serologic methods.(5)
Conference participants briefly discussed several salient properties of influenza viruses in general. Ultrastructurally, virions are spherical to pleomorphic and range from 80 to 120 nm in diameter, although filamentous forms may be much longer.(5) Germane to any review of influenza virus epidemiology is mention of the two mechanisms by which new variants of the virus emerge, forcing the continual need for vaccine reformulation to maintain population immunity: antigenic drift, accomplished through point mutations; and antigenic shift, resulting from genomic segment reassortment when two influenza viruses infect the same cell. Notably, both human and avian influenza viruses have established stable infections in pigs, which is attributed to the presence of receptors for both types of viruses on porcine epithelium; accordingly, pigs are regarded as a potential mixing vessel for the generation of pandemic influenza virus through reassortment.(4)
References:
2. Charlton BR, Chin RP, Barnes HJ: Fungal infections. In: Diseases of Poultry, ed. Saif YM, 12th ed., pp. 1005-1006. Blackwell Publishing, Ames, IA, 2008
3. Klasing KC: Nutritional diseases. In: Diseases of Poultry, ed. Saif YM, 12th ed., pp. 1128-1130. Blackwell Publishing, Ames, IA, 2008
4. Peiris JSM, de Jong MD, Guan Y: Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev 20:243-267, 2007
5. Swayne DE, Halvorson DA: Influenza. In: Diseases of Poultry, ed. Saif YM, 12th ed., pp. 153-184. Blackwell Publishing, Ames, IA, 2008
6. Tanimura N, Tsukamoto K, Okamatsu M, Mase M, Imada T, Nakamura K, Kubo M, Yamaguchi S, Irishio W, Hayashi M, Nakai T, Yamauchi A, Nishimura M, Imai K: Pathology of fatal highly pathogenic H5N1 avian influenza virus infection in large-billed crows (Corvus macrorhynchos) during the 2004 outbreak in Japan. Vet Pathol 43:500-509, 2006