Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 16
16 January, 2019
CASE I: FMVZ USP Case 16 (JPC 4019118).
Signalment: 2-month-old, female, Bernese Mountain Dog, Canis familiaris.
History: A 2-month-old female asymptomatic Bernese Mountain dog from a private kennel with sudden death few minutes after second vaccination using polyvalent vaccine (Recombitek C6/CV, Merial®) was sent to necropsy. The kennel’s vaccination protocol with Recombitek was: adults, yearly; puppies, beginning at 4 weeks of age with boosters after each 2 to 4 weeks until 12 weeks of age.
Gross Pathology: The puppy was in regular body condition, weighing 4Kg. The lungs were diffusely marked dark red, distended, edematous; trachea’s lumen contained transparent fluid. The liver was enlarged, diffuse dark red and friable. All the other organs were within normal limits of shape, size, color and consistency. The death cause being diagnosed as acute pulmonary insufficiency.
Laboratory results: None.
Microscopic Description:
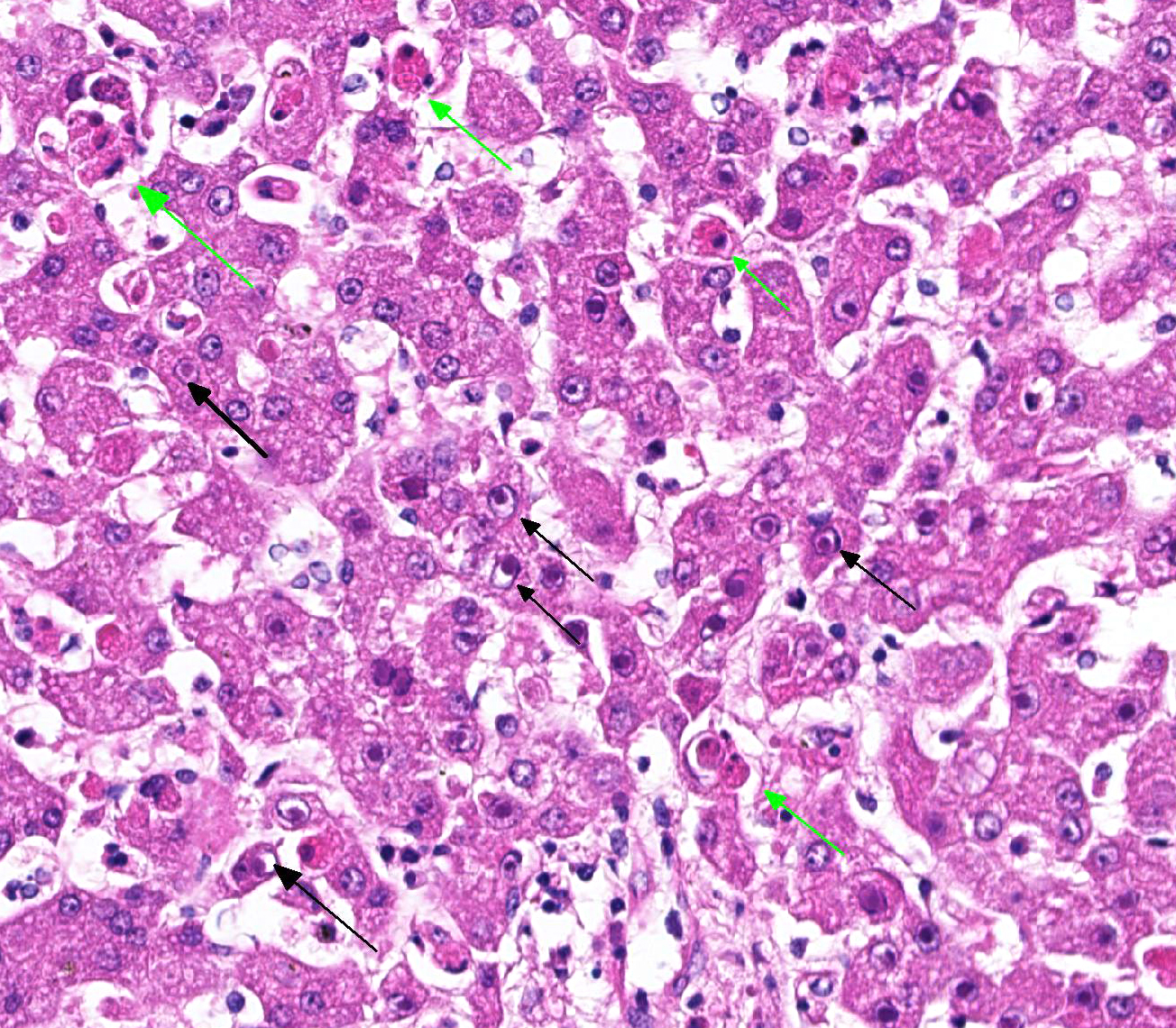
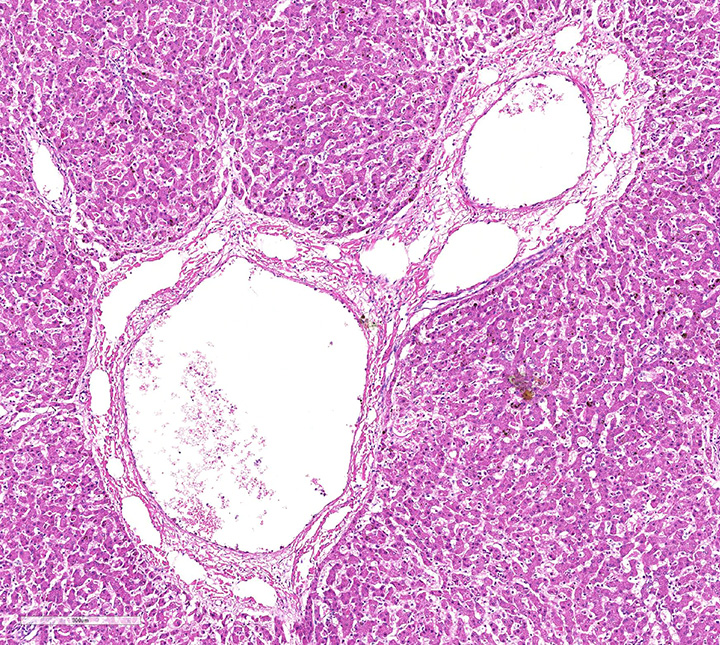
Histopathologic evaluation of the liver section showed mild disseminated periacinar (or centrilobular) necrosis and multiple midzonal foci of single-cell necrosis (apoptosis) of hepatocytes, with few neutrophils surrounding them. Numerous large eosinophilic intranuclear inclusion bodies are present in hepatocytes and in few Kupffer’s cells. Other findings include moderate congestion and diffuse mild microvesicular fatty change. The reticulin framework showed intact by reticulin stain; no changes were observed by picrossirius and Perls’ stain. The microscopical evaluation of lungs reveled acute diffuse hemorrhage, congestion and edema (lesion not showed).
Contributor’s Morphologic Diagnoses:
Liver: hepatitis, acute, neutrophilic, necrotic, periacinar, disseminated, mild with eosinophilic intranuclear inclusion bodies in hepatocytes and in Kupffer’s cells, etiology consistent with canine adenovirus 1, Bernese Mountain dog.
Contributor’s Comment: Canine adenoviruses (CAV) have been pathogens of dogs. There are two types, type 1 (CAV-1) and type 2 (CAV-2), which are responsible for infectious canine hepatitis (ICH) and infectious tracheobronchitis (ITB), respectively.2 CAV-1 causes infectious disease in dogs and other canids, has worldwide distribution and serologic homogeneity. The wild species affected are Canidae and Ursidae such as coyotes, foxes, wolves, and different species of bears. In addition, adenoviruses have been reported in otters (Lutra lutra) and marine mammals such as walruses (Odobenus rosmarus) and sea lions (Eumetopias jubatus)4. In Brazil the frequency of CAV infections in domestic dogs and wild canids is unknown.
Synonyms for ICH include epizootic fox encephalitis and Rubarth’s disease and it was first recognized in dogs in 1930. CAV-1 and CAV-2, a DNA virus, are members of the genus Mastadenovirus, family Adenoviridae, and are antigenically and genetically closely related (75% identity at the nucleotide level). Despite of this, they are easily distinguishable by restriction endonuclease analysis and DNA hybridization. They also exhibit different hemagglutination patterns and cell tropism: CAV-1 replicates in the vascular endothelial cells, hepatic and renal parenchymal cells, whereas CAV-2 replicates in the respiratory tract and to a limited extent in the intestinal epithelia.
Transmission occurs directly by animal-to-animal contact or indirectly through exposure to infectious saliva, feces, urine, or respiratory secretions. Viral spread occurs by contact of fomites and hands. Ectoparasites can harbor CAV-1 and may be involved in the natural transmission of the disease. The incubation period in dogs is 4 to 6 days after ingestion of infectious material and 6 to 9 days after direct contact with infected dogs. Viremia lasts 4 to 8 days postinfection and a antibody response clears the virus from blood and liver by day 7 postinfection and restricts the extent of hepatic damage. However, experiments show that dogs with partial neutralizing titer by day 4 or 5 postinfection may develop chronic active hepatitis and hepatic fibrosis. The mortality rate is 10% to 30%. Coinfections with canine coronavirus, canine distemper virus, or canine parvovirus can exacerbate the disease, increasing the mortality rates.
The virus enters the host through oronasal route and initially localizes in the tonsils, where it spreads to lymph nodes and lymphatics before reaching the bloodstream through the thoracic duct.3 The prime targets of viral localization and injury are hepatic parenchymal cells and vascular endothelial cells of many tissues, including the central nervous system (SNC). After 10 to 14 days postinoculation the virus can be found only in the kidneys and is excreted in the urine of carrier animals for up to 6 to 9 months. CAV-1 is highly resistant to environmental inactivation, surviving disinfection with chemicals such as chloroform, ether, acid, formalin and certain frequencies of ultraviolet radiation. It is inactivated after 5 minutes at 50oC to 60oC, which allows steam cleaning a mean of disinfection.3
Adenovirus is capable of infecting multiple organ systems; however, most infections are asymptomatic, and infections that result in disease may not be fatal. Clinical findings in the early phase of infection include increased rectal temperature (39.4oC to 41.1oC) and accelerated pulse and respiratory rates. Abdominal tenderness and hepatomegaly are usually apparent in the acutely ill dog and hemorrhagic diathesis may occur. Depression, disorientation, seizures or terminal coma can develop at any time after infection. Severely affected dogs die within a few hours after the onset of clinical signs, while mildly affected dogs may recover after the first febrile episode. Classic symptoms, such as corneal edema and anterior uveitis (“blue eye”), occur when clinical recovery begins and may be the only clinical abnormalities seen in dogs with unapparent infection. Death cause of ICH is uncertain. Although the liver is a primary site of viral injury some dogs die so suddenly that liver damage with resulting hepatic failure does not occur. Death in these dogs can result from damage to the brain, lungs, and other vital parenchymatous organs, or from the development of disseminated intravascular coagulation (DIC) during the early viremic phase of the disease.
Corneal opacity (“blue eye”) and interstitial nephritis may occur 1 to 3 weeks after recovery because of deposition of immune complexes. Hematologic findings include leukopenia (<2000 cells/lL of blood; mainly attributable to a decrease in neutrophil count), increase in the serum transaminases (only in the severe forms of disease), and coagulation disorders associated with disseminated intravascular coagulation (DIC; thrombocytopenia, altered platelet formation, and prolonged prothrombin time). Proteinuria (albuminuria) can easily reach values greater than 50 mg/dL because of immunomediated glomerulonephritis. Icterus is uncommon in acute ICH, but it is found in some dogs that survive the acute fulminant phase of the disease.
Necropsy and biopsy of liver from dogs can usually confirm a diagnosis of ICH. During acute phase dogs are often in good flesh, with edema and hemorrhage of superficial lymph nodes and cervical subcutaneous tissue. Blotchy hemorrhages may be present on the serous membranes, as well as a small quantity of fluid in the abdomen. The liver is slightly enlarged, with sharp edges, turgid and friable, sometimes congested and spotted with small round areas of necrosis; the gallbladder appears thickened, edematous, and grayish or bluish white opaque in color. Edema of the gallbladder wall is a constant finding. Red strands of fibrin can be found on its capsule, especially between the lobes. Intraluminal gastrointestinal hemorrhage is a frequent finding. Gross lesions in other organs are inconstant and include multifocal hemorrhagic renal cortical infarcts, areas of pulmonary consolidation and edematous bronchial lymph nodes. The brain can be slightly swollen and on cut surface multifocal petechial hemorrhage and gray discoloration of brainstem can be observed. The Bernese puppy necropsy showed only enlarged and friable liver and edematous and dark red lungs which means that the puppy was in acute phase of CAV 1 infection.
Histopathologic changes in the liver of dogs that died of acute hepatitis include widespread centrilobular (periacinar) to panlobular necrosis and individual hepatocellular necrosis, along with neutrophilic and mononuclear cell infiltration and intranuclear inclusions in the hepatocytes and Kupffer’s cells. Fatty changes are common but not constant. Multifocal areas of congestion, hemorrhage, and leukocyte infiltration can be observed in several organs, mainly in the lymphoid organs and kidneys, because of vascular damage and inflammation. Interstitial nephritis and iridocyclitis with corneal edema are also present in dogs recovering from ICH. Viral inclusions are initially found in the renal glomeruli and later in renal tubular vascular endothelium. Lymphoid follicles are dispersed with central areas of necrotic foci. The lungs have thickened alveoli with septal cell and peribronchial lymphoid accumulations. Alveoli in consolidated areas are filled with an exudate consisting of erythrocytes, fibrin, and fluid. Swollen, desquamated endothelial cells in meningeal vessels contain intranuclear inclusions. Mononuclear cuffing is present around small vessels throughout the parenchyma of the CNS. Mild endothelial proliferation and mononuclear perivascular infiltration persist for at least 3 weeks after clinical recovery. The histopathologic specificity of the lesions is based on the demonstration of large, intranuclear inclusion bodies. They can be also found in vascular endothelial cells and histiocytes.
The Bernese puppy had no previously symptoms and died quickly after second vaccination. The CAV 1 infection diagnosis was made based on characteristic microscopic liver lesions. The death cause was acute pulmonary insufficiency consistent with anaphylaxis, a vaccine-associated adverse event and CAV infection was considered a finding not directly correlated to dead The lung lesions were interpreted as immediate hypersensitivity reaction to polyvalent vaccine that overlapped virus infection. Generally, in the dog, clinical signs of type I hypersensitivity include facial edema (“big head”), pruritus, hypotensive shock, weakness, dyspnea, and vomiting with or without diarrhea that can be hemorrhagic. Local or systemic reactions can occur in young puppies within 1 to 24 hours after their second or third vaccination and can result in acute clinical signs such as previously described, and death.
The ICH diagnosis can be helped by hematologic findings (eg, leukopenia, prolonged blood clotting, increased activities of alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), although the increase of transaminases is commonly observed only in severely affected or moribund dogs. Postmortem findings and histopathologic changes are highly consistent of CAV-1 infection when the inclusion bodies, which have been classified as Cowdry type A, are present in both ectodermal and mesodermal tissues. Confirmation of a diagnosis of ICH is obtained by virus isolation on permissive cell lines, such as Madin Darby canine kidney (MDCK) cells.2 A polymerase chain reaction (PCR) protocol has recently been developed for molecular diagnosis. Ocular swabs, feces, and urine can be collected in vivo for virus isolation and PCR. Postmortem samples can be withdrawn from the kidney, lung, and lymphoid tissues. The liver is rich in arginase, which inhibits viral growth in cell cultures, but it represents the most important organ for histopathologic examination. Viral growth in cells is revealed by rounding cells that form clusters and detach from the monolayer. Immunofluorescence (IF) can detect viral antigens in infected cell cultures and in acetone-fixed tissue sections or smears. Viral replication can also be demonstrated by detection of nuclear inclusion bodies in the cells after hematoxylin-eosin staining. Neither virus isolation nor IF is able to distinguish between the two adenovirus types.2 Because CAV-2 can also be detected in the internal organs and feces of vaccinated or acutely infected dogs and CAV-1 is also frequently isolated from respiratory secretions, trachea, and lungs, distinction between CAV-1 and CAV-2 necessarily deserves laboratory examination. Restriction fragment length polymorphism analysis on viral genomes using the endonucleases PstI and HpaII generates differential patterns. Detection and differentiation of CAV-1 and CAV-2 by PCR with a single primer pair are also possible.2 Although CAVs agglutinate erythrocytes of several species, hemagglutination is not used in routine diagnosis. Because most dogs are vaccinated and since CAV-2 infection is frequent in dogs, serology has low diagnostic relevance.
Vaccination has controlled the disease and turned it rare in domestic dog population, although severe outbreaks can be still observed in countries in which CAV vaccines are not used routinely or as a consequence of uncontrolled importation of dogs from endemic areas. Clinical management of dogs that develop ICH is primarily symptomatic and supportive expecting hepatocellular repair. Vaccination usually is repeated yearly, although after administration of two doses of CAV-2 vaccine, immunity seems to persist for more than 3 years. Even extensive vaccination has greatly reduced the incidence of CAV infections, re-emergence of ICH has been described in some countries such Italy, probably do to trading of pups with uncertain sanitary status from Eastern Europe. At the moment, there are few data on the molecular epidemiology of CAVs, but it is commonly accepted that vaccine breaks occur rarely with CAV vaccines, because the viruses are genetically stable. Accordingly, CAV infection in vaccinated dogs has been associated with maternally derived antibody (MDA) interference in the early life of the pups rather than with emergence of variants genetically distant from the prototype strains contained in CAV-2 vaccines.
We assumed first vaccination failure in the Bernese puppy due to maternally derived antibody interference. Regarding that maternally derived antibody (MDA) titers decrease below 100 around 5 to 7 weeks of age .Sporadic cases in which dogs do not get adequate vaccination during puppyhood are still seen. The duration of passively acquired immunity in the pup is dependent on the antibody concentration of the bitch. The half-life of CAV-1 antibodies is 8.6 days, and these values correlate well with the half-life for canine globulin. The recommended schedule with any vaccine for protection against ICH involves at least two doses, given 3 to 4 weeks apart, at 8 to 10 and 12 to 14 weeks of age.
The intensification of surveillance activity using new diagnostic techniques and molecular analysis tools may help to investigate the epidemiology of CAV infections more thoroughly and plan adequate measures of control in different countries.
Contributing Institution:
Faculdade de Medicina Veterinária e Zootecnia da Universidade de São Paulo
Av. Prof. Dr. Orlando Marques de Paiva, 87
CEP 05508 270
Cidade Universitária
São Paulo/SP – Brasil
JPC Diagnosis: Liver: Hepatitis, necrotizing, multifocal, centrilobular to midzonal, mild to moderate, with edema and numerous hepatocytic and endothelial intranuclear viral inclusions.
JPC Comment: The contributor has provided an excellent and wide-ranging discussion of canine adenovirus-1 in the dog and in a wide range of other species.
A purported viral disease that shows a number of similarities to infectious canine hepatitis was identified in Great Britain in 1985, but may not be well-known (or even heard of by WSC participants. “Canine acidophil cell hepatitis” gets its name from the shrunken necrotic hepatocytes scattered throughout the liver, but lacks the intranuclear inclusions and CAV-1 and CAV-2 have not been identified. Transmission studies of liver homogenates induced reproducible disease, suggesting a viral agent which has not yet been characterized. Also, unlike infectious canine hepatitis, the chronic disease characterized by cirrhosis and in some cases hepatocellular carcinoma have been identified in suspect cases.3
References:
- Caudell D, Confer AW, Fulton RW, et al. Diagnosis of infectious canine hepatitis virus (CAV-1) infection in puppies with encephalopathy. J Vet Diagn Invest. 2005; (17):58-61.
- Decaro N, Martella V, Buonavoglia C. Canine adenoviruses and herpesvirus. Vet Clin Small Anim 2008; (38): 799–814.
- Greene CE. Infectious Canine Hepatitis and Canine Acidophil Cell Hepatitis. In: Greene CE, ed. Infectious Diseases of the dog and cat. 4th ed. Philadelphia, PA: Saunders; 2011:42-47.
- Greene, CE, Levy, JK. Immunoprofilaxy. In: Greene CE, ed. Infectious Diseases of the dog and cat. 4th ed. Philadelphia, PA: Saunders; 2011:1163-1205.
- Stalker MJ, Hayes MA. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 5th ed. Vol 2. New York, NY: Elsevier Saunders; 2007:348-351.
- Thompson H, O´Keeffe AM, Lewis JCM, et al. Infectious canine hepatitis in red foxes (Vulpes vulpes) in the United Kingdom. Veterinary Record, 2010; (166)111-114.
- Watson PJ. Chronic hepatitis in dogs: a review of current understanding of the aetiology, progression, and treatment. The Veterinary Journal. 2004; (167)228-241.