WSC 2022-2023
Conference 12
Case I
Signalment:
4 years-old, female, goat (Capra hircus), unspecified breed.
History:
The sample was submitted by a general practitioner and little information is available regarding the clinical history. This goat was pregnant and was one month away from parturition. Anorexia was reported.
Gross Pathology:
Kidneys are described by the practitioner as pale and swollen, with irregular white foci. Other reported findings are mesenteric lymph nodes with a caseous and mineralized content. Only kidneys were submitted for histopathological analysis.
Laboratory Results:
No findings reported.
Microscopic Description:
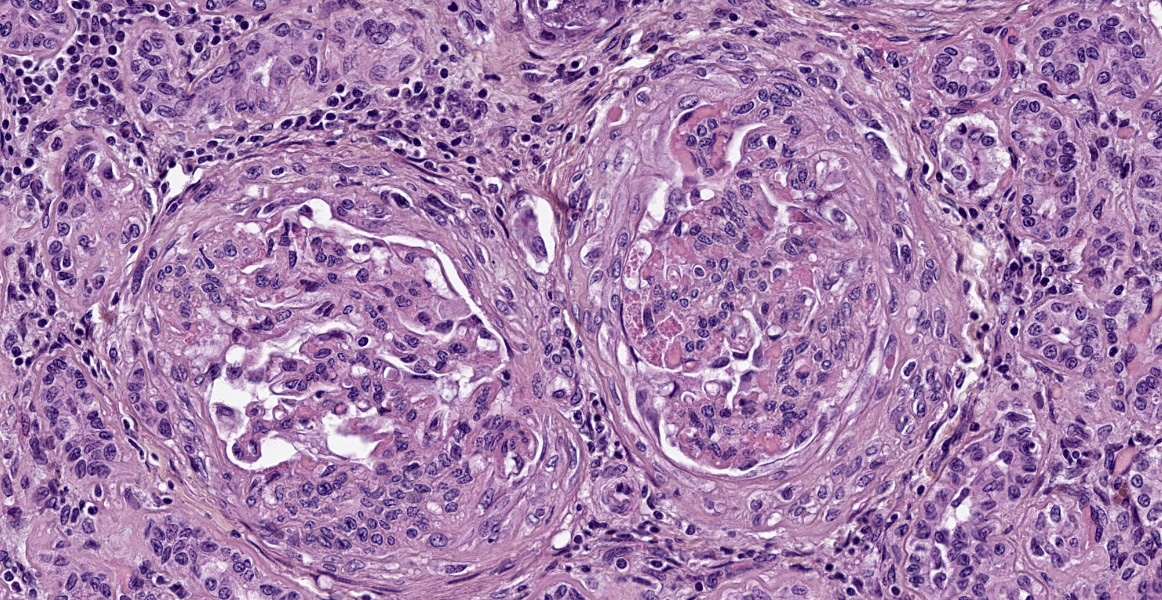
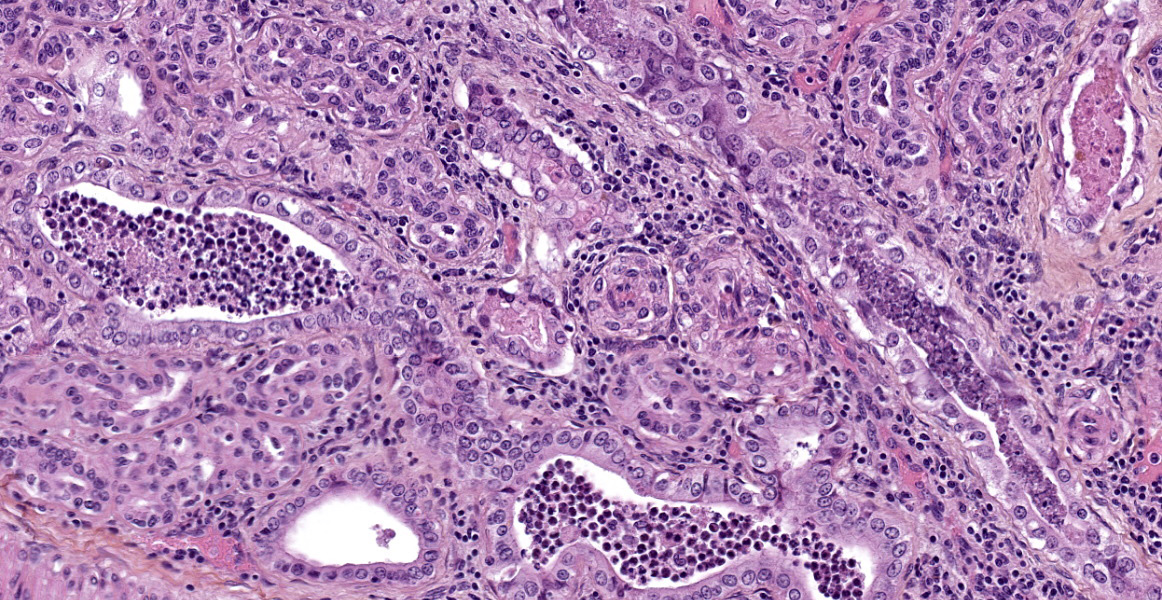
Almost all glomeruli are affected by one or more of the following changes:
- Variable thickening of the glomerular capsule with fibroblastic proliferation;
- Marked thickening of glomerular basement membrane with moderate increased in mesangial cellularity (membranoproliferative glomerulonephritis);
- Adhesions between the glomerular tufts and the capsule (glomerular synechiae);
- Extraglomerular proliferation of spindle cells admixed with fibrin around glomerular tufts, resembling glomerular crescents;
- Glomerular congestion and/or hemorrhages;
- Eosinophilic granular materiel (protein exudation) and/or hemorrhages within the urinary space;
- Shrunken and fibrotic glomeruli (global glomerulosclerosis).
These changes affect almost all glomeruli (diffuse changes) and usually the entire glomerular tuft (global changes).
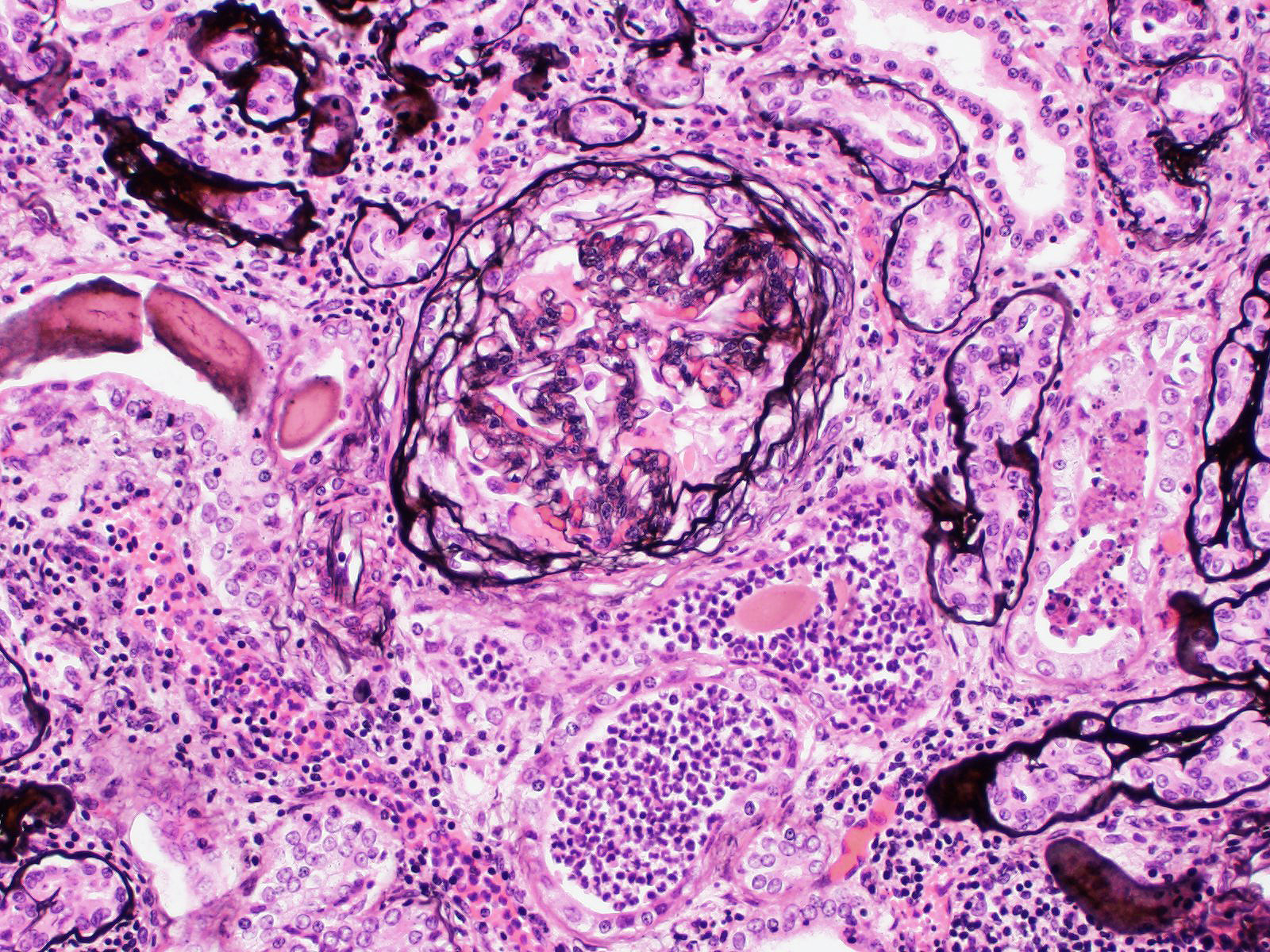
Also affected are tubules showing a combination of changes: thickened basement membrane; epithelial atrophy; tubular dilation with eosinophilic protein casts, degenerate neutrophils and/or eosinophilic granular debris; mineralization of basement membranes, especially in the medulla; rare crystals (likely calcium oxalate crystals).
The interstitium is moderately expanded by fibrosis and/or infiltration by lymphocytes and plasma cells.
Contributor’s Morphologic Diagnoses:
Glomerulonephritis, membranoproliferative, diffuse, global, marked, chronic, with global glomerulosclerosis, lymphoplasmacytic interstitial nephritis, tubular atrophy and tubular casts.
Contributor’s Comment:
The case is interesting as it shows a constellation of renal elementary lesions. Glomerulonephritides can be defined as primary glomerular diseases with secondary tubulointerstitial and vascular changes.2,4 In this case, the proportion of affected glomeruli (more than 50%), the proportion of the glomerular tuft affected (entire glomerular tuft) and the type of glomerular change (thickening of glomerular basement membrane with mesangial proliferation) favor a diagnosis of diffuse, global, membranoproliferative glomerulonephritis1. An interesting feature is the presence of crescent-like structures around glomerular tufts, that are reminiscent of crescentic glomerulonephritis in humans. Such crescents have been described in cases of glomerulonephritis in ruminants.2,3
Among glomerular diseases, glomerulonephritis is frequent in veterinary medicine and are the subject of a rather complex nomenclature, based on human renal pathology that can be difficult to apply routinely to veterinary pathology cases especially when additional techniques (immunofluorescence, electron microscopy) are not available. The exact cause is generally unknown but immune-complex deposition secondary to infections is a likely cause in most cases. In ruminants, Maedi-Visna virus and Bovine Viral Diarrhea virus are suspected to initiate immune-mediated glomerulonephritis.2
Despite showing marked renal changes of glomeruli, tubules and interstitium, there was no clinical sign or gross lesion suggestive of renal failure in this animal. Clinically silent glomerulonephritis appears to be common in ruminants with the exception of the membranoproliferative glomerulonephritis of Finnish landrace sheep that is present at birth and caused by deficiency in complement component C3. Affected lambs die of renal failure around 1-3 months.2
Contributing Institution:
Ecole Nationale Vétérinaire d’Alfort
Unité d’Histologie et d’Anatomie Pathologique, BioPôle Alfort
Département des Sciences Biologiques et Pharmaceutiques
7 avenue du Général De Gaulle
94704 Maisons Alfort Cedex
FRANCE
www.vet-alfort.fr
JPC Diagnosis:
- Kidney: Glomerulonephritis, membranoproliferative, chronic, diffuse, severe with crescent formation and periglomerular and interstitial fibrosis.
- Kidney: Tubular degeneration and necrosis, multifocal, moderate with numerous tubular casts and intratubular crystals.
JPC Comment:
Immune complex deposition can cause different types of glomerular disease depending on where they are deposited. In general, deposition of immune complexes in the glomerulus leads to complement activation, membrane attack complex formation, mast cell degranulation, and leukocyte chemotaxis.2 Activated monocytes and neutrophils cause secondary enzymatic and oxidative damage to podocytes, endothelial cells, and the mesangium.2 Mesangial cells can also produce inflammatory mediators such as IL1 and arachidonic acid metabolites, initiating inflammation and cause self-induced proliferation.2 The end result is an amplifying loop of inflammation and proliferation.2
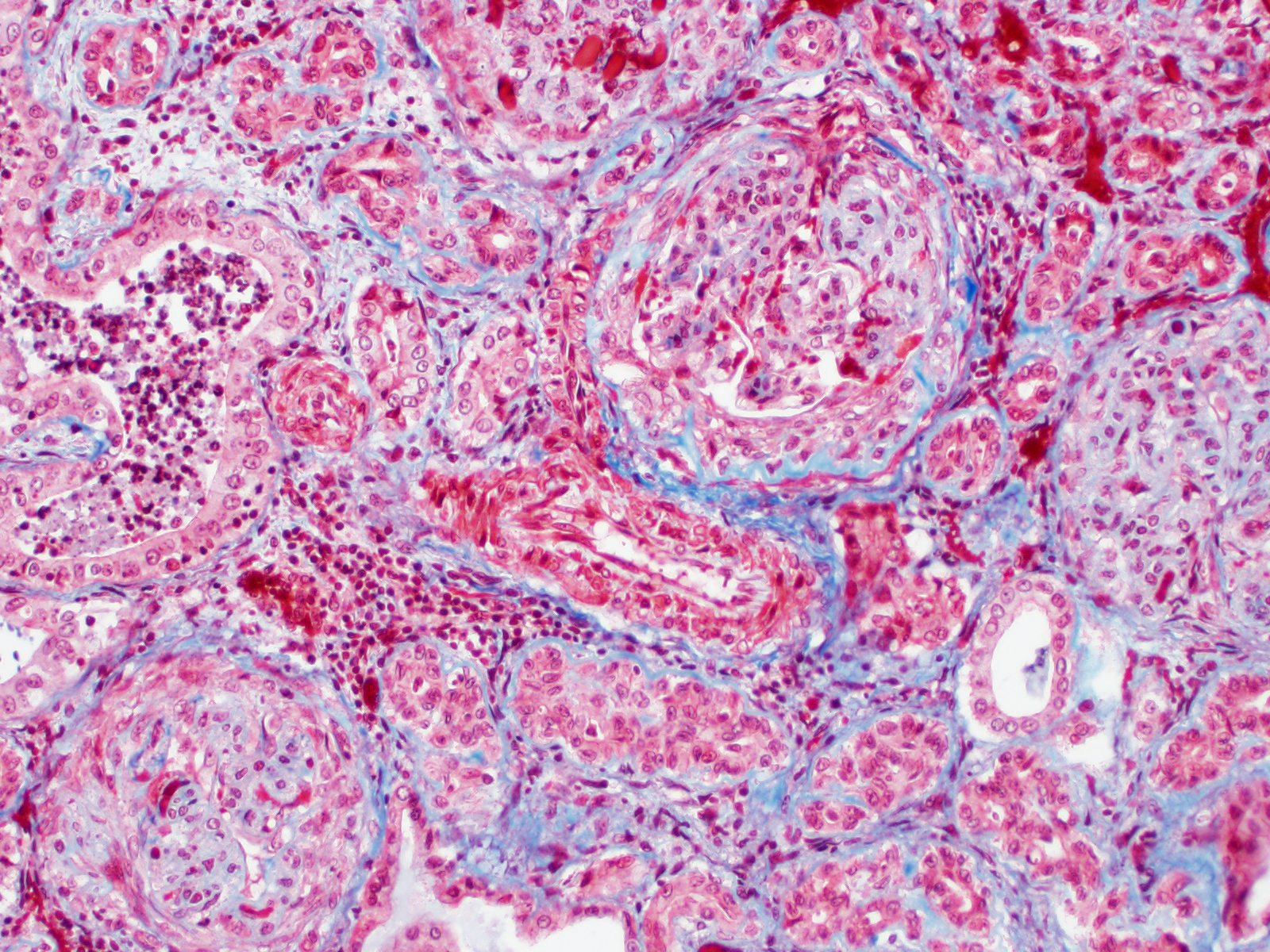
As the contributor mentions, electron microscopy and/or immunofluorescence are necessary for confirming immune complex deposition and differentiating it from other conditions with similar histologic appearances. Special stains such as silver stains (i.e. Jones methenamine silver), PAS, and Masson’s trichrome may be helpful for visualizing glomerular basement membrane changes.1
Deposition of immune complexes between podocytes and the glomerular basement membrane leads to membranous glomerulonephropathy.1 Changes are minimal early in the disease. Later in disease, glomerular basement membranes are thickened, and in advanced stages, there may be secondary glomerulosclerosis.1 Remodeled glomerular basement membrane features spikes and holes which can be visualized with silver stains such as JMS.1
Immune complex deposition on the luminal surface of capillaries leads to membranoproliferative glomerulonephritis.1 Subsequent endothelial hypertrophy, mesangial hypertrophy, and/or the presence of leukocytes which characteristic hypercellularity of the endocapillary and mesangial compartments.1 Glomerular basement membranes may be thickened or duplicated, which JMS staining demonstrates in this case.1 Secondary changes that may occur with membranoproliferative glomerulonephritis include glomerular synechiae and crescents, as seen in this case.1
Immune complex deposition within the mesangium leads to mesangioproliferative glomerulonephritis, and resulting hypercellularity is limited to the mesangium.1
While low levels of calcium oxalate crystals in the kidney may be an incidental finding in any species, the moderator, Dr. Alicia Moreau, and conference participants remarked about the prevalence of crystals within the section. Ruminants may ingest oxalates in plants such as halogeton, greasewood, rhubarb, and sorrel/dock, or as a mycotoxin in moldy feed.5 In general, metabolism of oxalate in the rumen makes ruminants resistant to formation of oxalate calculi, but both low magnesium diet and low calcium diets can favor formation of oxalate urolithiasis.5 Oxalate crystals form in renal vessels and tubules and can cause obstruction, trauma, and necrosis of tubular epithelium, which conference participants suspected in this case.5 Proximal convoluted tubules are most susceptible to injury due to their high metabolic activity.5
References:
- Cianciolo R, Brown C, Mohr C, et al. Atlas of Renal Lesions in Proteinuric Dogs. The Ohio State University; 2018.
- Cianciolo RE, Mohr FC. Urinary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th Elsevier, 2017; 401-413, 456-457.
- Ohnuki T. Crescentic glomerulonephritis induced in the goat by immunization with homologous or heterologous glomerular basement membrane antigen. Acta Pathol Jpn. 1975;25(3):319-331.
- Slauson DO, Lewis RM. Comparative Pathology of Glomerulonephritis in Animals. Vet Pathol. 1979;16(2):135-164.
- Sula M, Lane LV. The Urinary System. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th Elsevier, 2022; 721,734, 736