CASE II: R18-1576 (JPC 4137580)
Signalment:
Approximately 1-2 month old, female, Nile tilapia, Tilapia niloticus, fingerling, fish
History:
Mortalities of 10-30% occurred over a four-month period in a tilapia hatchery receiving untreated surface water. Reported clinical signs were limited to lethargy, erratic swimming, and exophthalmia.
Gross Pathology:
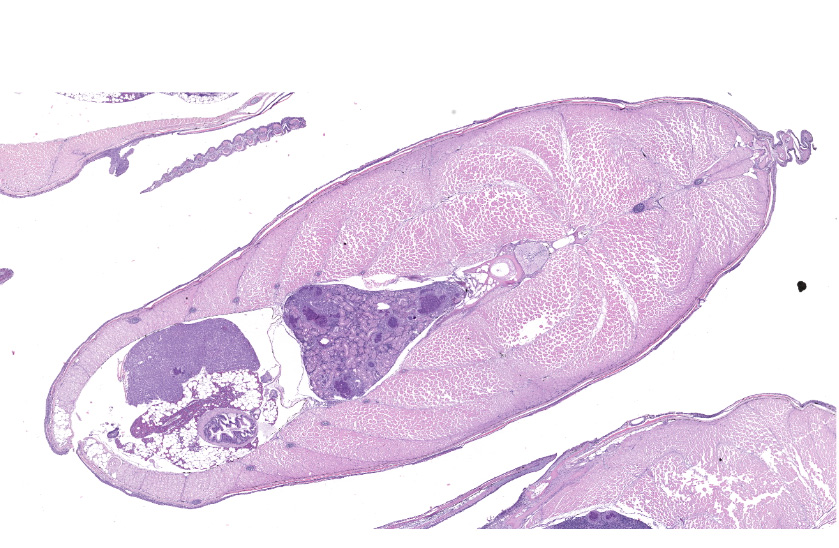
Exophthalmia was accompanied by severe renomegaly and splenomegaly, with pinpoint white nodules widely distributed throughout.
Laboratory Results:
Pure growth of Edwardsiella anguillarum was cultured from the brains of multiple fish and its identity confirmed by specific end-point PCR. Ancillary testing for tilapia lake virus (TiLV) and Francisella noatunensis subsp. orientalis was negative.
Microscopic Description:
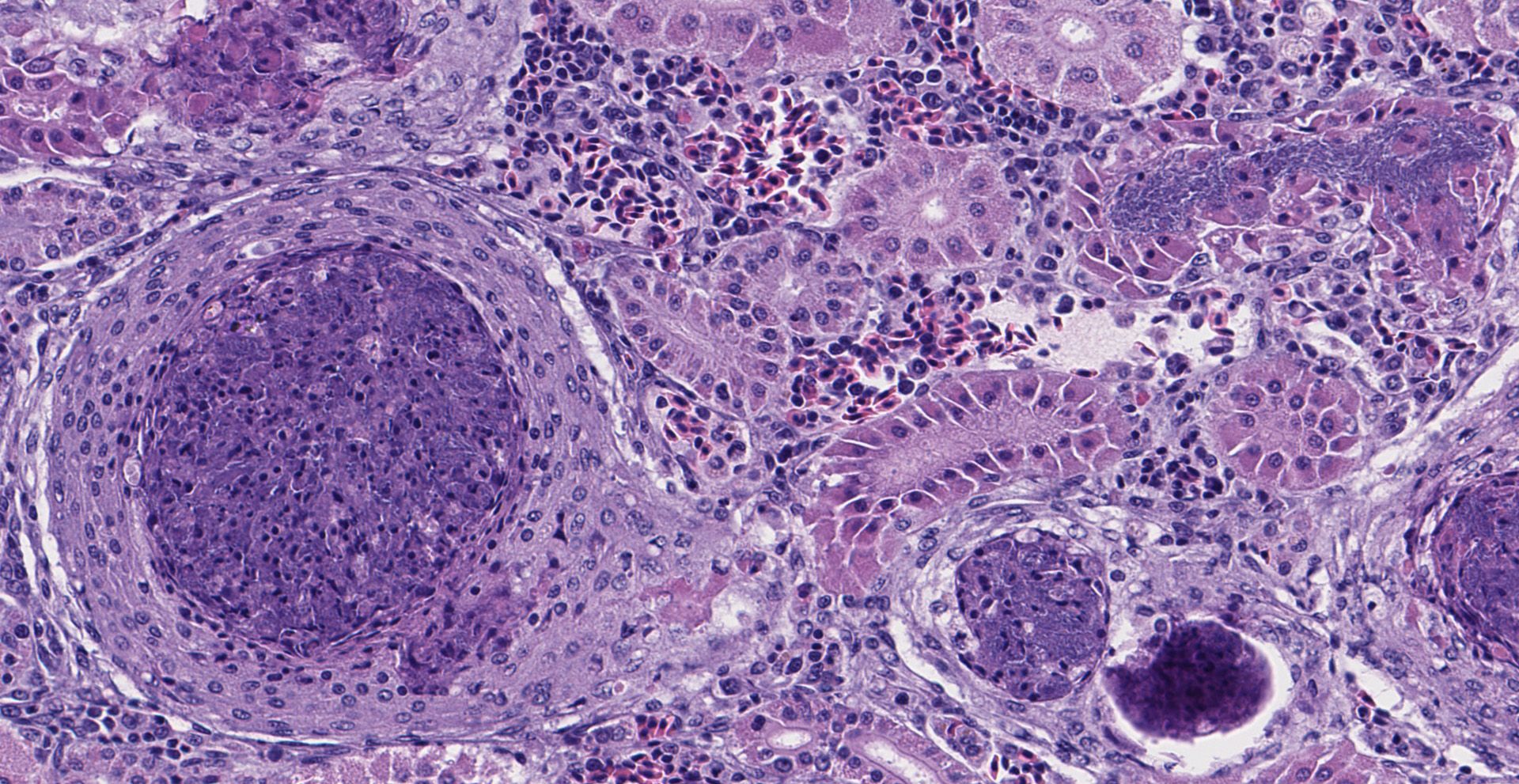
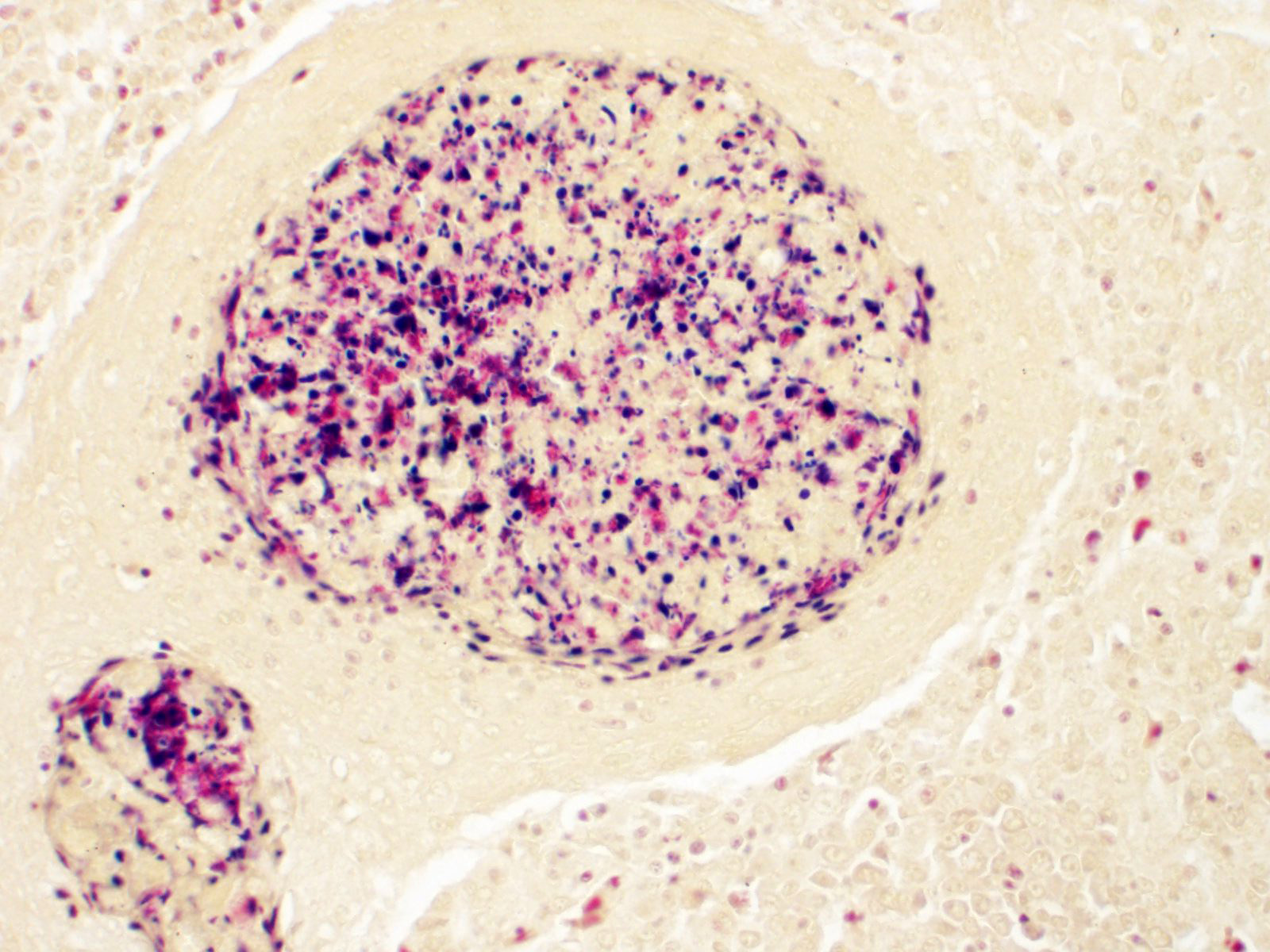
Multifocal to occasionally coalescing, expansile granulomas replace hematopoietic tissue and displace adjacent tubules within the anterior region of the kidney. Central regions of granulomas are formed by extensive areas of hypereosinophilic, pyknotic, and karyorrhectic cellular debris, mixed with degenerate macrophages containing large numbers of intracytoplasmic bacterial rods. Broad mantles formed by concentric layers of epithelioid macrophages limit granulomas. Large numbers of free bacterial rods mixed with necrotic epithelial cell debris fill scattered tubular lumens. A single, similar granuloma is present in the liver.
Contributor's Morphologic Diagnoses:
Kidney: Epithelioid granulomas, multifocal, subacute to chronic, severe, and scattered tubular necrosis, with free and intracytoplasmic gram-negative bacterial rods.
Contributor's Comment:
This kidney section from a tilapia was from one of 20 fish received from a group of hatchery-reared fingerlings experiencing chronic mortalities. Microscopic findings were consistent with systemic Edwardsiella anguillarum infection, an emerging fish pathogen with an expanding host range.5 Edwardsiella anguillarum is a facultative intracellular, gram-negative, bacterial rod originally described from diseased eel (Anguilla spp.) in China.11 In the United States, identification dates back to samples of striped bass (Morone saxatilis) archived in 1994.10 Multi-organ necrosis and granuloma formation, with intracellular bacterial rods, characterizes lesions.2 Although variable in distribution, severity, and chronicity among individuals, similar lesions affected the meninges, intracranial adipose, ventricular system of the brain, olfactory nerves, ocular choroid rete, heart, gills, pseudobranch, spleen, kidney, liver, gastrointestinal wall, swim bladder rete, skeletal muscle, and various connective tissue sites among the various tilapia.
Lesions are reported to be most severe in highly vascular organs, particularly spleen and kidney, but can be widely disseminated and affect almost any tissue site. Early lesions are necrotizing, containing abundant cellular debris, variable numbers of macrophages, and large numbers of 3-5 µm, free and phagocytized, gram-negative, bacterial rods. Over time, thin margins of attenuated and epithelioid macrophages envelop foci of necrosis, ultimately forming granulomas with large central areas of hypereosinophilic cellular debris bordered by wide zones of epithelioid macrophages. Bacteria can be observed in H&E sections, but are best visualized with Giemsa stain. They occur primarily within macrophage cytoplasm, consistent with the facultative intracellular nature of Edwardsiella spp. bacteria. With chronicity, bacterial numbers decline sharply and may not be discernable in mature granulomas.2
The genus Edwardsiella contains the well-known bacterial fish pathogens, E. ictaluri and E. tarda, as well as the two recently recognized species, E. piscicida and E. anguillarum, that were split from E. tarda.1,11 Edwardsiella ictaluri is best known as the agent of enteric septicemia of catfish (ESC), an important disease of catfish aquaculture in the southeastern United States.5 Edwardsiella tarda affects both terrestrial and aquatic hosts, and has been implicated in disease outbreaks in numerous economically important fish species worldwide.8 However, the validity of many E. tarda identifications from fish is now questionable.5 Similar to E. anguillarum, E. piscicida appears to exhibit little host specificity and is increasing in importance as a global pathogen.3,5 Due to shared phenotypic characteristics and high 16S rRNA sequence homology, differentiation of E. tarda, E. piscicida, and E. anguillarum require species-specific PCR methods or evaluation of additional gene sequences (e.g. gyrB) for correct identification.5,10 Current MALDI-TOF databases may not recognize the latter two species.
In addition to E. anguillarum, disease outbreaks in tilapia have been attributed to E. tarda, E. ictaluri, and E. piscicida, although not all E. tarda identifications may be reliable.4,5,10,12 Lesions described particularly for E. piscicida and E. ictaluri resemble those seen here, suggesting the respective diseases may not be reliably differentiated by histopathology, which should be supported by appropriate bacterial culture and molecular testing.2,3 Additional differential diagnoses include mycobacteriosis and francisellosis caused by Francisella noatunensis subsp. orientalis.
Contributing Institution:
Department of Pathology
College of Veterinary Medicine
University of Georgia
Athens, GA 30602
JPC Diagnosis:
1. Kidney: Granulomas, multiple, with multifocal renal tubular degeneration, necrosis, and loss, and colonies of bacilli.
2. Liver: Granuloma, focal.
3. Skin: Epidermitis and dermatitis, erosive to ulcerative, moderate, multifocal, with Trichodina sp.
JPC Comment:
Family Enterobacteriaceae is composed of facultative anaerobic, non-spore forming, gram-negative rods which ferment glucose and a variety of other sugars, are oxidase negative, and catalase positive. Over 28 genera have been identified and include major pathogens such as Escherichia, Salmonella, and Yersinia, as well as opportunists such as Proteus, Klebsiella and Serratia.10
In the early 1960s, a unique group of isolates that did not fit into known genera were identified from both a human patient with enteric fever and from snakes in Japan with acute gastroenteritis. The isolates were subsequently identified as a new genus and species, Edwardsiella tarda. Additional species have since been identified and include E. ictaluri, E. hoshinae, E. piscicida, and E. anguillarum, infecting a wide range of hosts including freshwater and marine fish, reptiles, avians, and mammals. As noted by the contributor, multiple studies over the first half of the previous decade found the species previously identified as E. tarda was composed of three genetically distinct but phenotypicically identical species: E. tarda, E. piscicida, and E. anguillarum.11
Historically, E. tarda has been identified as a zoonotic pathogen that can be acquired from both fish (including ornamental pet fish) and shellfish. A range of clinical manifestations of E. tarda have been reported in humans and include necrotic dermal lesions, gastroenteritis, and septicemia in severe cases that results in osteomyelitis, meningitis, or cholecystitis. Given the recent discoveries of E. piscicida and E. anguillarum, it is unclear if these previous reports are attributable to E. tarda or a newly recognized species.11
Studies conducted prior to the discovery of E. piscicida and E. anguillarum found multiple virulence factors affiliated with E. tarda's pathogenesis. EypP is an effector protein always present in virulent strains whereas avirulent strains lack the gene encoding for EypP. Additional important virulance factors include AIDA, EthA, EthB, Fur, QseB, and QseC. AIDA is an autotransporter adhesin that facilitates attachment and penetration into host cells. Both EthA and EthB are hemolysins.6 Ferric Uptake Regulator (Fur) is a transcription factor responsible for the regulation of the pathogen's intracellular iron concentration, which is inherently protective against the formation of reactive oxygen species generated by the Fenton reaction.6,14 Both QseB and QseC are responsible for phenomenon known as quorum sensing, which plays an important role in the pathogen's cellular invasion and intracellular survival, particularly within macrophages, by both regulating virulence factors as well as surface structure modifications (e.g. flagella and fimbriae) which may facilitate adaptation to the intracellular environment.6
Although Edwardsiella ictaluri is commonly associated with catfish and tilapia, it has also been reported in aquarium species including the green knifefish (Eigenmannia virescens), rosy barb (Puntius conchonius) and zebrafish (Danio spp.). Zebrafish are particularly susceptible to a genetically distinct strain from those infecting catfish and tilapia, which has destroyed between 20,000-40,000 fish. Given zebrafish are commonly utilized in the research setting, this pathogen has the capacity to significant impact studies. However, infection is typically detected during quarantine prior to research facility entry. Although in vitro studies have shown E. ictaluri to be susceptive to multiple antibiotics, neither bath nor oral antibiotic administration have been shown to be effective in vivo.7
A secondary finding in this case are multifocal regions of erosive to ulcerative dermatitis associated with superficial parasitic ciliates consistent with Trichodina sp. Speciation based on histologic examination alone is difficult due to high intraspecific variation, high interspecific similarity, and low host specificity.15 All clinically important species affect the skin and/or gills and both marine and freshwater fish are commonly infested.8
Trichodinosis is typically a mild disease resulting in chronic morbidity or mortality, but significant losses may occur, particularly with young fish and those with secondary bacterial infections. Trichodinids exhibit a characteristic scooting motion on the tissue surface and can be diagnosed using a wet mount of skin or gill or histologically, however, these organism are easily lost during the fixation process.8
When viewed from the dorsal aspect, trichodinids typically measure 40-60µm (up to 120µm) in diameter and are characterized by a round shape, a central ring of hook-like denticles, and circumferential peripheral cilia.8
Trichodinids most commonly infest debilitated fish, such as in this case. Therefore, particularly in cases of low numbers (e.g. one per 100X field of view), a primary underlying disease process should be ruled out in cases of trichodinosis.8
References:
1. Abayneh T, Colquhoun D, Sørum H. Edwardsiella piscicida sp. nov., a novel species pathogenic to fish. J Appl Microbiol. 2013;114:644-654.
2. Armwood AR, Camus AC, López-Porras A, Ware C, Griffin MJ, Soto E. Pathologic changes in cultured Nile Tilapia (Oreochromis niloticus) associated with an outbreak of Edwardsiella anguillarum. J Fish Dis. 2019; DOI: 10.1111/jfd.13058
3. Camus A, Griffin M, Armwood A, Soto, E. A spontaneous outbreak of systemic Edwardsiella piscicida infection in largemouth bass Micropterus salmoides (Lacépède 1802) in California, USA. J Fish Dis. 2019;42:759-763.
4. El-Seedy F, Radwan I, Abd E-G, Sayed H. Phenotypic and genotypic characterization of Edwardsiella tarda isolated from Oreochromis niloticus and Clarias gariepinus at Sohag Governorate. J Am Sci. 2015;11: 68-75.
5. Griffin MJ, Greenway TE, Wise D. Edwardsiella spp. In: Woo P, Cipriano R, eds. Fish Viruses and Bacteria: Pathobiology and Protection. Wallingford, UK: CABI; 2017: 190-210.
6. Katharios P, Kalatzis PG, Kokkari C, Pavlidis M, Wang Q. Characterization of a Highly Virulent Edwardsiella anguillarum Strain Isolated From Greek Aquaculture, and a Spontaneously Induced Prophage Therein. Front Microbiol. 2019;10:141.
7. Kent ML, Sanders JL, Spagnoli S, Al-Samarroe CE, Murray KN. Review of diseases and health management in zebrafish Danio rerio (Hamilton 1822) in research facilities. J Fish Dis. 2020;43:637-650.
8. Noga EJ, ed. Problems 11 through 43. In: Fish Disease Diagnosis and Treatment. 2nd ed. Ames, IA: Wiley-Blackwell; 2010:137-138.
9. Park SB, Aoki T, Jung TS. Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish. Vet Res. 2012;43:67.
10. Quinn PJ, Markey BK, Leonard FC, FitzPatrick ES, Fanning S, Hartigan PJ. Enterobacteriaceae. In: Veterinary Microbiology and Microbial Disease. 2nd ed. Ames, Iowa: Blackwell Science Ltd; 2011:107-127.
11. Reichley SR, Ware C, Steadman J, et al. Comparative phenotypic and genotypic analysis of Edwardsiella isolates from different hosts and geographic origins, with emphasis on isolates formerly classified as E. tarda, and evaluation of diagnostic methods. J Clin Microbiol. 2017;55:3466-3491.
12. Shao S, Lai Q, Liu Q, et al. Phylogenomics characterization of a highly virulent Edwardsiella strain ET080813T encoding two distinct T3SS and three T6SS gene clusters: Propose a novel species as Edwardsiella anguillarum sp. nov. Syst Appl Microbiol. 2015;38:36-47.
13. Soto E, Griffin M, Arauz M, Riofrio A, Martinez A, Cabrejos ME. Edwardsiella ictaluri as the causative agent of mortality in cultured Nile tilapia. J Aquat Anim Health. 2012;24:81-90.
14. Troxell B, Hassan HM. Transcriptional regulation by Ferric Uptake Regulator (Fur) in pathogenic bacteria. Front Cell Infect Microbiol. 2013;3:59. 00059
15. Wang Z, Liu M, Ma H, et al. Redescription and molecular characterization of two Trichodina species (Ciliophora, Peritrichia, Mobilida) from freshwater fish in China. Parasitol Int. 2022;86:102470.