CASE III:
Signalment:
Male NSG (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) mouse. Age unknown.
History:
The colony was recently treated with trimethoprim sulfamethoxazole for an outbreak of diarrhea. Several animals have been found dead or presented moribund.
Gross Pathology:
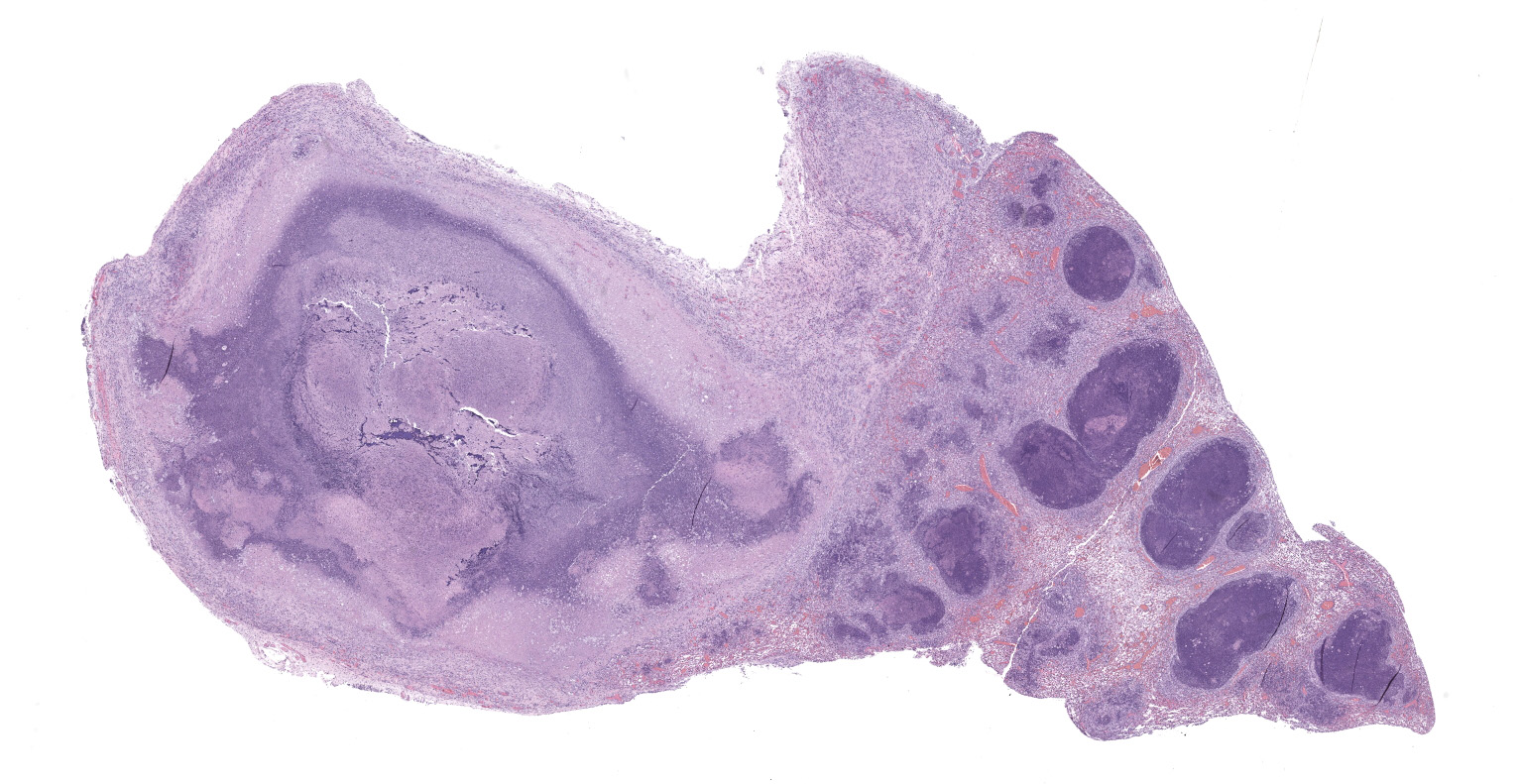
The mouse was in poor body condition with scant amounts of visceral fat. The left cranial lung lobe was effaced by a 1 X 0.8 X 0.8 cm mass (abscess) containing some white to yellow/green material (pus), with multifocal nodules throughout the rest of the left lung. Right lung was not affected.
Laboratory Results:
Bacterial culture of the lungs returned Klebsiella pneumoniae (string test negative).
Microscopic Description:
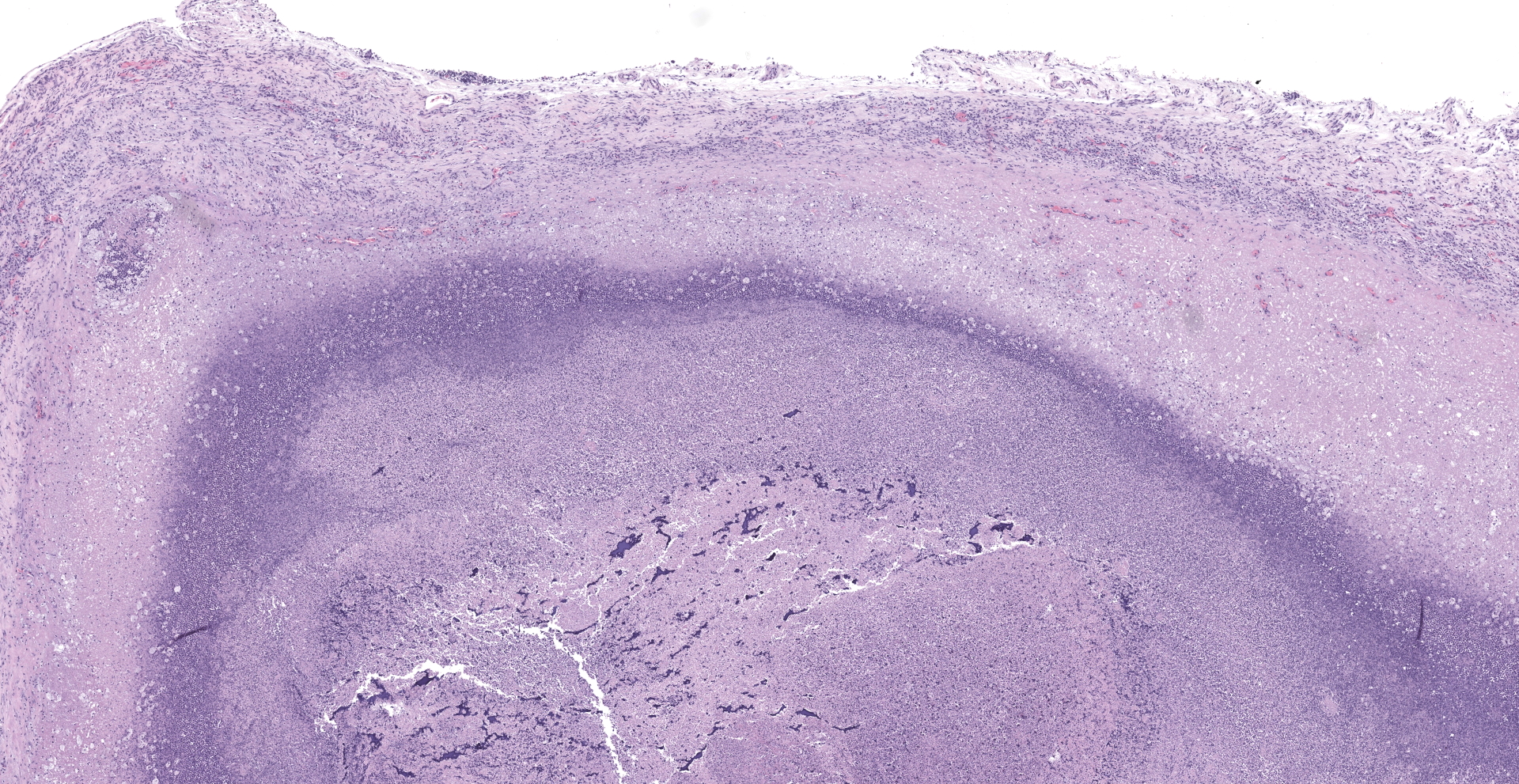
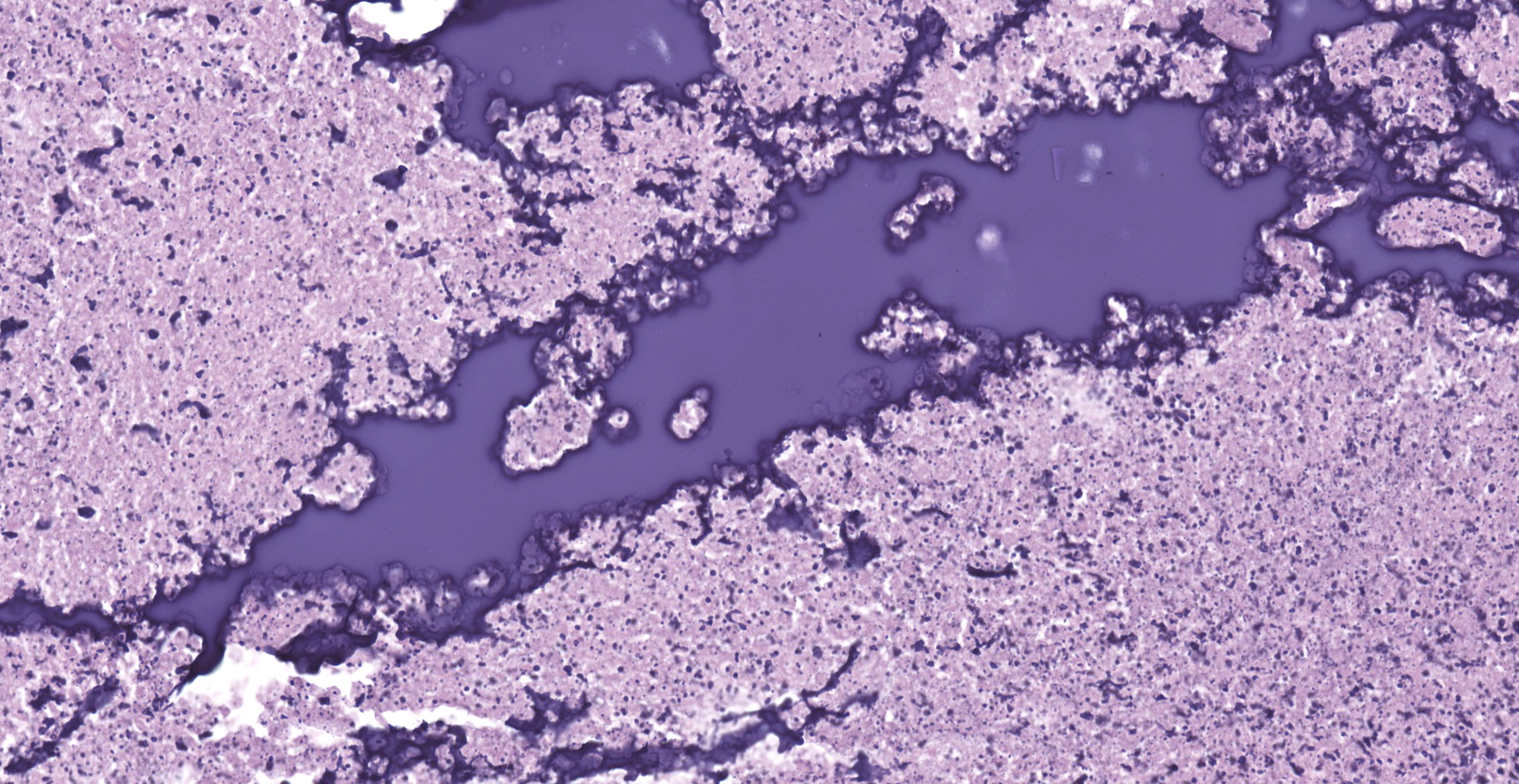
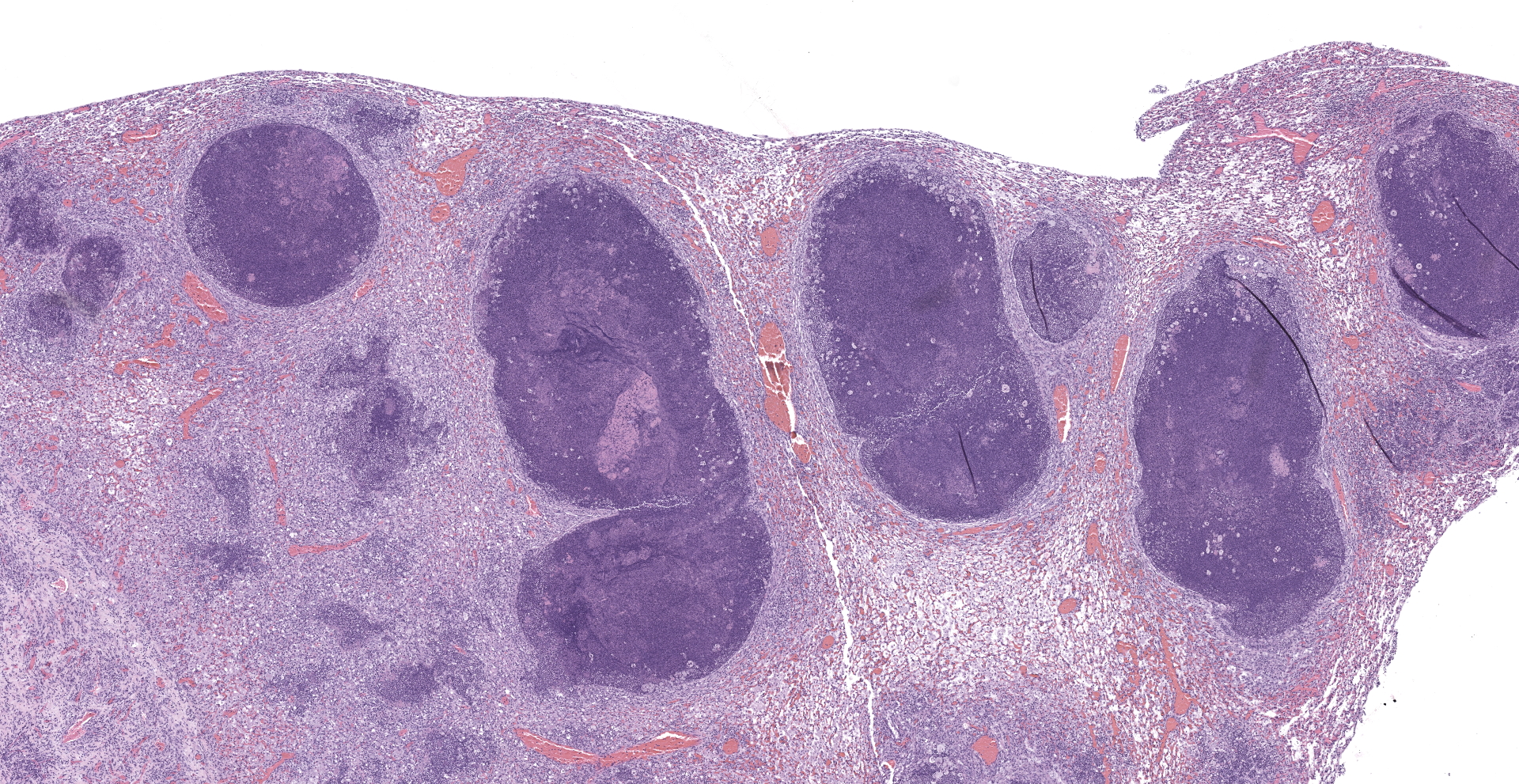
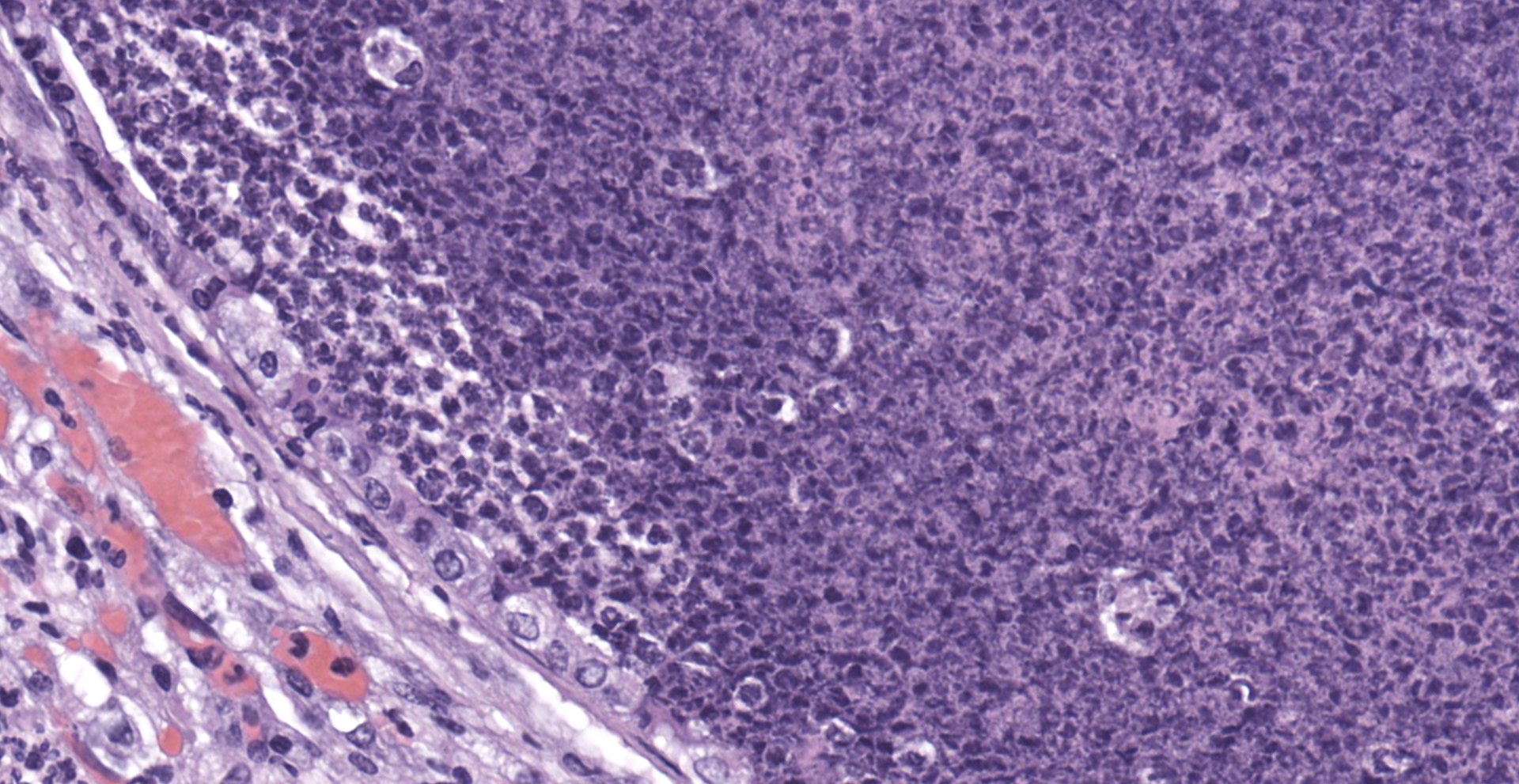
Lung: The submitted lung section consists of a large expansile abscess that severely effaces the pulmonary parenchymal architecture as well as multiple discrete foci of severely distended bronchioles plugged with basophilic necro-purulent and proteinaceous exudative material. The abscess is composed of large core of necrotic and proteinaceous material surrounded by massive numbers degenerate neutrophils, karyorrhectic debris, basophilic lytic chromatin and mineralized material admixed with multifocal aggregates of intralesional bacilli. The periphery of the abscess is composed of a variable thick band of collagen fibers admixed with hypertrophic stromal cells, numerous large foamy macrophages and abundant neovascularization. The affected bronchioles, are filled with basophilic concretions of large numbers of degenerate neutrophils, ghost cells, karyorrhectic debris, fibrin deposition, aggregates of bacilli, with few dispersed, eosinophilic, linear to rhomboid crystalline material. There is also marked attenuation of bronchiolar epithelium or loss of epithelium lining. There is expansion and splitting of the bronchial walls by edema and associated inflammation. Most of the alveoli between these dilated bronchioles are filled with aggregates of degenerate neutrophils, proteinaceous material along with prominent alveolar septal necrosis, type II pneumocyte hyperplasia, edema, vascular congestion, and mild hemorrhage. In less affected areas, the alveolar space is occupied by a few foamy macrophages, and multinucleated giant cells are also rarely noted.
Contributor's Morphologic Diagnoses:
1. Lung: Severe, multifocal to coalescing, acute, necrotizing fibrinosuppurative bronchopneumonia, with pulmonary abscessation, septal necrosis, type II pneumocyte hyperplasia, pulmonary edema, congestion, and intralesional bacilli.
2. Mild, multifocal, chronic, histiocytic pneumonia, with intraalveolar eosinophilic crystals, lung, mouse.
Contributor's Comment:
This case presents a classic Klebsiella pneumoniae lesion: bacterial pneumonia with extensive lytic necrosis.1 This mouse also had a severe multifocal fibrinosuppurative meningitis (not shown), a lesion commonly seen in human patients.6 Klebsiella pneumoniae is a common commensal bacterial species found in the gastrointestinal tract of healthy mice, but can be an opportunistic pathogen especially for immunocompromised mice.6 In recent years the emergence of hypervirulent strains, characterized by their thick capsule which produces a hyperviscomucoid phenotype (as identified using the "string test") have emerged as important pathogen in nosocomial and community acquired infections in humans.8 The strains of Klebsiella pneumoniae isolated from this colony of the present case were overwhelmingly "classical" or non-hypervirulent.
The presented mouse came from a colony, which were previously treated with TMP/SMX for an outbreak of diarrhea; many of the Klebsiella pneumoniae strains isolated from this colony were resistant to this antibiotic; Klebsiella pneumoniae commonly contain multiple antibiotic resistance genes.7 While it is unclear in this case what role the antibiotic treatment played, it is well established that antibiotic induced dysbiosis can allow the overgrowth of normal microbiome species, leading to disease.1 This mouse, along with several in this room, also had a high burden of trichomonads, later confirmed on PCR to be Tritrichomonas muris. These organisms are normally thought of as non-pathogenic and was considered an incidental finding.1
NSG (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) mice contain several critical mutations (NOD/ShiLtJ background with severe combined immune deficiency mutation (scid) and an interleukin-2 (IL-2) receptor gamma chain deficiency). These mice do not have functional T and B cells, NK cells, and hemolytic complement; they lack cytokine signaling due to deficiency of IL-2 gamma chain as the common receptor.3 Because of profound immunodeficiency, NSG mice have been widely used for research of xenotransplantation, serving as "humanized mice".3 Severe immunocompromised condition also put NSG mice in high risk of opportunistic bacterial infections. A report has revealed that spontaneous mortality had occurred in NSG mice, and 10-20% of mortality was caused by nephritis associated with Enterococcus sp. or Klebsiella oxytoca, both of which were opportunistic pathogens.3 Previously, there have been case reports with thorough reviews regarding Klebsiella pneumonia in CyBB knockout mouse (Mus musculus, JPC 4101147) and Klebsiella-induced meningoencephalitis in NSG mouse (Mus musculus, JPC 4128009). This case will be a great comparison for previous cases.
In humans, the K. pneumoniae infection could turn into serious nosocomial diseases, also known as Klebsiella-associated syndromes (KAS). The pathological manifestations are liver abscesses, pneumonia, and bacteremia, potentially meningoencephalitis and endophthalmitis.2,5 In non-human primates, K. pneumoniae infection is not common. A study in 20205 revealed that a total number of 9 predominantly outdoor-housed rhesus macaques (Macaca mulatta) presented for pneumonia (n=6), meningoencephalitis (n=7), endophthalmitis/optic neuritis (n=3), liver abscess (n=2), and other nonspecific findings such as gastric rupture (n=1), endometriosis (n=1), amyloidosis in liver/kidneys/intestines (n=2), thyroid tumor (n=1), and colonic adenocarcinoma (n=1). These animals tested positive for K. pneumonia through PCR or bacterial culture, from tissues of lung, brain, bone marrow, pleura, and peritoneum.5 Another study showed that 7 African green monkeys (Chlorocebus aethiops) presented multisystemic abscesses in abdominal cavity or in lungs, with positive bacterial culture of K. pneumonia with a hyperviscous mucoid phenotype in the string test.10
There are very few case reports of K. pneumonia in other animal species. A case report revealed 7 California Sea Lions (Zalophus californianus) presented with severe purulent bronchopneumonia, fibrinonecrotizing pleuritis, and pyothorax, and infection of hypervirulent (hyperviscomucoid) strain of K. pneumoniae was confirmed through serotyping, PCR, sequencing, and immunohistochemistry.9
Klebsiella pneumonia posted a zoonotic potential, since the bacteria shares the common molecular/phenotypes between humans and animals.11 Eosinophilic crystalline pneumonia, also known as acidophilic macrophage pneumonia (AMP), is a common background lesion in lab animals. The eosinophilic crystals are composed of Ym1 and Ym2 chitinase, with iron, alpha-1 antitrypsin, immunoglobulin, and fragments of granulocytes. 4
Contributing Institution:
The Division of Comparative Medicine, Massachusetts Institute of Technology
https://comp-med.mit.edu/
JPC Diagnosis:
Lung: Bronchopneumonia, necro-suppurative, multifocal to coalescing, severe, with abscessation.
JPC Comment:
The contributor provides an excellent review of manifestations of Klebsiella sp. infection reported in various species.
Klebsiella pneumoniae is a gram-negative, non-motile, encapsulated, facultative anaerobic, non-spore forming, non-flagellated, bacillus in the Enterobacteriaceae family. K. pneumoniae has numerous virulence factors, including (but not limited to) its capsule, lipopolysaccharide (LPS), siderophores, and fimbriae (also known as pili).
The capsule is composed of an extracellular polysaccharide matrix that surrounds the bacteria. Classic strains of K. pneumoniae produce capsules with serotypes K1 to K78, with K1 and K2 being more pathogenic. Highly virulent (HV) strains amplify the production of capsular material, resulting in a hypercapsule, and are predominately of the K1 serotype with fewer in the K2 serotype. The capsule significantly contributes to K. pneumoniae's pathogenesis by inhibiting phagocytosis, hindering the bactericidal action of antimicrobial peptides such as β-defensins and lactoferin, preventing complement-mediated lysis and opsonization by blocking the binding of complement (e.g. C3), and downregulates the immune response by decreasing reactive oxygen species, IL-8, IL-6, and TNF-α production via activating a NOD-dependant pathway and shielding LPS recognition by TLR receptors. Enhanced capsule production may occur in HV strains in multiple ways, including expression of mucoid phenotype A (rmpA) and rmpB as plasmid-borne transcription regulators, chromosomal expression of rmpA, and regulation of the capsule synthesis A and B genes (rcsA and rcsB). In addition, hypercaspule production may also be initiated independantly by the chromosomal mucoviscosity-associated gene A (magA), which is restricted to K1 strains.8
LPS is a component of gram-negative bacteria and is composed of three major components: lipid A, an oligosaccharide core, and O antigen. Lipid A is connected to the bacteria's membrane and is a well-known pathogen associated molecular pattern recognized by TLR4. TLR4 activation leads to the recruitment of neutrophils and macrophages via production of cytokines and chemokines. However, multiple strains of K. pneumoniae evade TLR4 activation through various mechanisms, including masking of LPS with the capsule (K1, K10, and K16 strains), dampening TLR4 signaling using the capsule, or potentially modify LPS into a no longer recognizable form. LPS remodeling has been reported to occur in other bacteria such as Yersinia pestis and Helicobacter pylori.7
The O antigen of LPS also protects the bacterium from complement mediated destruction. Although K. penumoniae is recognized by the classical, alternative, and lectin complement pathways, strains with the full length O antigen (or "smooth LPS") are protected from the effects the membrane attack complex by binding complement (i.e. C3b) far away from the cell membrane, rendering the membrane attack complex ineffective. Strains with a truncated or absent O antigen (or "rough LPS") are more susceptible to killing via the classical complement pathway due to the increased ability for C1q to bind to the cell's surface.8
Fimbriae are a common feature of almost all members of Enterobacteriaceae, including K. pneumoniae, which has both type 1 and type 3 fimbriae. Type 1 fimbriae are composed of FimA subunits with the FimH subunit at the tip. These filamentous, membrane-bound, adhesive structures facilitate K. pneumoniae's ability to colonize the urinary tract in addition to biofilm formation. However, type 1 fimbriae are also immunogenic, as they amplify lectinophagocytosis by macrophages and neutrophils while the FimH subunit also binds to mast cells, resulting in increased immune cell activation and recruitment of neutrophils. Interestingly, K. pneumoniae appears to regulate the expression of type 1 fimbriae genes as they're expressed within the urinary tract but not in the gastrointestinal tract or lungs. Type 3 fimbriae are helix-like, membrane bound, adhesive structures that are notably required for the formation of biofilm production and adhesion on medical devices such as endotracheal tubes, giving the bacteria access to the lungs.8
The fourth major virulence factor utilized by K. pneumoniae is its use of siderophores to scavenge iron from the host. As with many bacteria, the pathogen requires iron in order to thrive, which correlates to iron sequestration by the host as a component of the innate immune response. Under normal conditions, iron is typically bound to transport molecules such as transferrin; however, mammalian species shift iron to lactoferrin, an innate defense protein, during bacterial infections. Siderophores possess higher affinities for iron than host transport proteins and also have the ability to liberate iron from chelating proteins while also scavenging iron from the environment. K. pneumonia has been found to produce one or more of the following siderophores: enterobactin aerobactin, salmochelin, and yersiniabactin. Nearly all classical and HV strains of K. pneumoniae produce enterobactin, which is considered to the primary iron uptake system utilized by this species.8
K. pneumoniae possesses many additional virulence factors, including outer membrane proteins (OMPs), porins, efflux pumps, and transporters, which in combination with the previously described factors impart a high level of resiliency to this pathogen, making it particularly well suited to resist both host immune defenses as well as therapeutic intervention.8
Conference attendees discussed the significance of eosinophilic crystals consistent with acidophilic macrophage pneumonia (MAP). It is unclear if MAP is simply a background lesion or developed secondary to the severe bronchopneumonia observed in this case. In addition, most participants noted bacteria consistent with K. pneumoniae were inapparent in their sections, highlighting the importance of bacterial culture in regard to the determination of an etiologic diagnosis in similar cases.
References:
1. Barthold, S., Griffey, S., Percy D. Pathology of Laboratory Rodents and Rabbits, Fourth Edition. Chichester: John Wiley & Sons, Inc.; 2016.
2. Büyüktuna SA, Hasbek M, Çelik C, et al. Yoğun Bakım Ünitesinde Gelişen Klebsiella pneumoniae Enfeksiyonları: Karbapenem Direnci ve Hasta Mortalitesi ile İlgili Risk Faktörler. Mikrobiyol Bul. 2020 15;54:378?391.
3. Foreman O, Kavirayani AM, Griffey SM, Reader R, Shultz LD. Opportunistic Bacterial Infections in Breeding Colonies of the NSG Mouse Strain. Vet Pathol. 2011 3;48:495?499.
4. Hoenerhoff MJ, Starost MF, Ward JM. Eosinophilic crystalline pneumonia as a major cause of death in 129S4/SvJae mice. Vet Pathol. 2006 Sep;43(5):682-8
5. Keesler RI, Colagross-Schouten A, Reader JR. Clinical and Pathologic Features of Spontaneous Klebsiella pneumoniae Infection in 9 Rhesus Macaques ( Macaca mulatta ). Comp Med. 2020 1;70:183?189.
6. Ku Y-H, Chuang Y-C, Chen C-C, et al. Klebsiella pneumoniae Isolates from Meningitis: Epidemiology, Virulence and Antibiotic Resistance. Sci Rep. 2017 26;7:6634.
7. Kumar V, Sun P, Vamathevan J, et al. Comparative Genomics of Klebsiella pneumoniae Strains with Different Antibiotic Resistance Profiles. Antimicrob Agents Chemother. 2011 55:4267?4276.
8. Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol Mol Biol Rev. 2016 80:629?661.
9. Seguel M, Gottdenker NL, Colegrove K, Johnson S, Struve C, Howerth EW. Hypervirulent Klebsiella pneumoniae in California Sea Lions (Zalophus californianus): Pathologic Findings in Natural Infections. Vet Pathol. 2017 11;54:846?850.
10. Twenhafel NA, Whitehouse CA, Stevens EL, et al. Multisystemic Abscesses in African Green Monkeys ( Chlorocebus aethiops ) with Invasive Klebsiella pneumoniae ?Identification of the Hypermucoviscosity Phenotype. Vet Pathol. 2008 45:226?231.
11. Yang F, Deng B, Liao W, Wang P, Chen P, Wei J. High rate of multiresistant Klebsiella pneumoniae from human and animal origin. Infect Drug Resist. 2019 12:2729?2737.