Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 24
10 April, 2019
CASE III: S643/17 (JPC 4101752)
Signalment: Foal, 3 weeks of age, female, crossbred horse, Equus caballus
History: This foal was born without any complication in March 2017 and developed normal the following days. At the third week of life, the animal was put with other horses and foals on the pasture. The following morning, the foal was lethargic and dyspneic with a body temperature of 40 °C. A veterinarian was called in immediately. The foal was treated with NSAIDs. The body temperature declined within 30 minutes. Another 30 minutes later, the foal showed tachycardia (100/min), reduced breathing rate (3/min) with cyanosis of mucous membranes and died spontaneously. All other horses and foals in the flock were healthy the entire time.
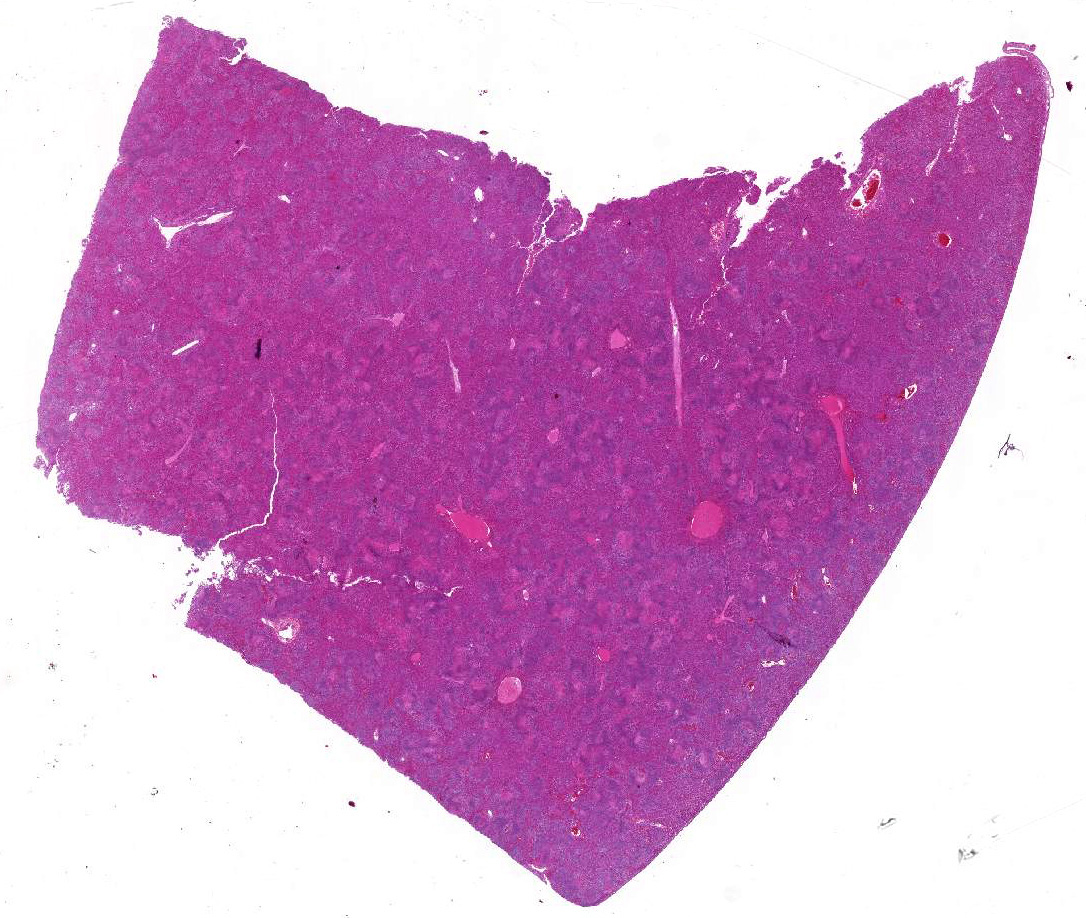
Gross Pathology: Postmortem examination was performed immediately the same morning. The foal was in good body condition and developmental stage. A marked jaundice was visible with intensely yellowish staining of mucous membranes, connective tissue and fasciae as well as the intima of large vessels. The liver was severely enlarged, mottled reddish brown with a friable consistency. White spots were not visible within the parenchyma. The spleen was mildly enlarged. Beside alveolar edema and emphysema of the lungs, other organs and tissues including heart, brain, thymus, umbilicus, and joints were unremarkable macroscopically.
Laboratory results: Virology (detection of cytopathogenic viruses in cell culture): negative
Bacteriology (aerobic and anaerobic culture): numerous colonies of E. coli and Pseudomonas sp. in all organs, few colonies Clostridium perfringens in the small intestine, and unspecific bacteria (e.g. Acinetobacter johnsonii, aerobic Bacillus sp., Enterobacter ludwigii, Providencia rettgeri) in liver, lung, intestine, spleen and brain
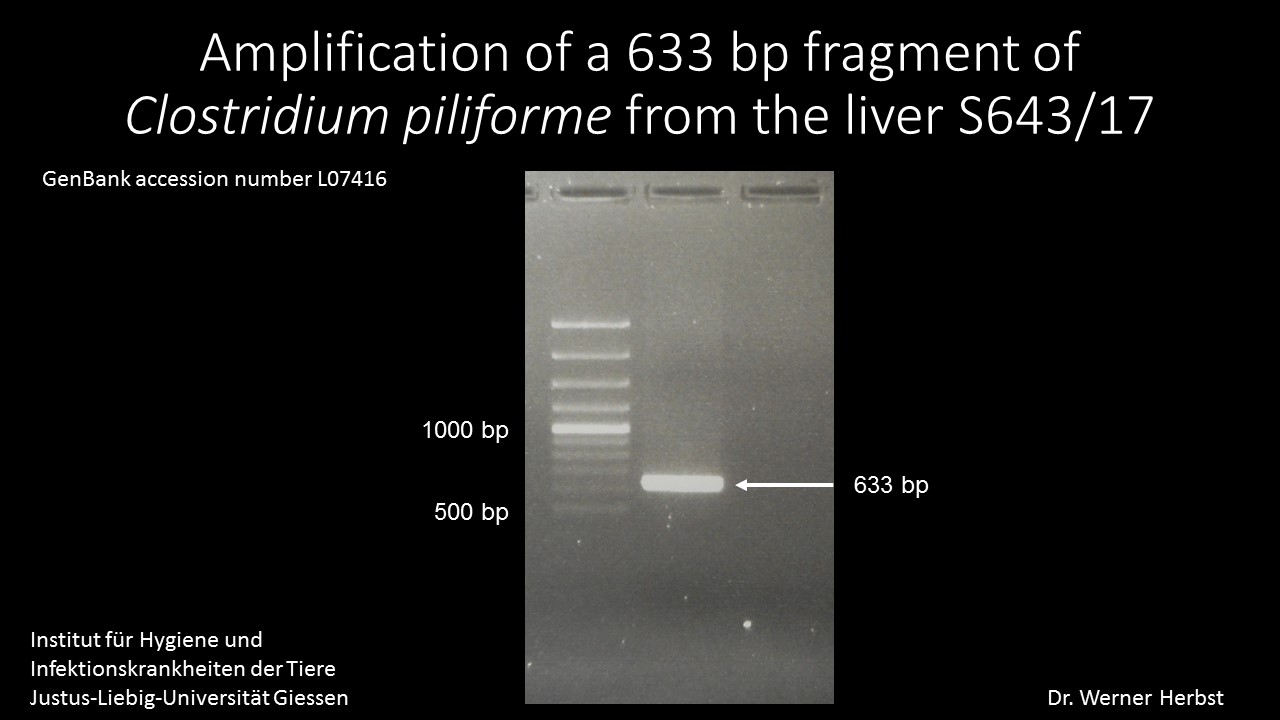
PCR/liver: Amplification of a 633 bp fragment of Clostridium piliforme (GenBank accession number L07416) (Fig.1)
Microscopic Description:
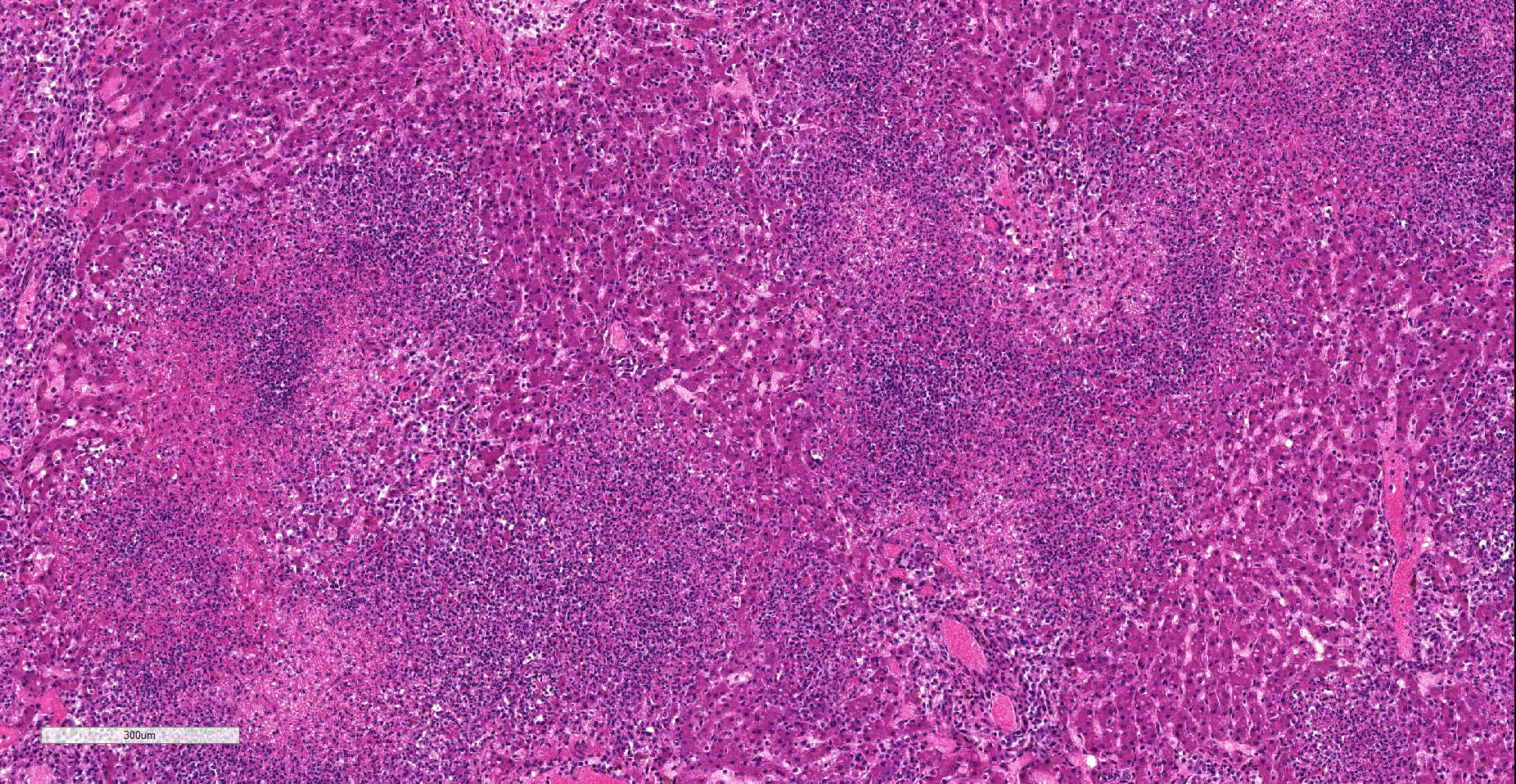
Liver: Affecting 80% of hepatic parenchyma are multifocally to coalescing and randomly distributed areas of lytic necrosis characterized by loss of cellular detail and replacement of hepatocytes by nuclear and cellular debris admixed with fibrillar to beaded eosinophilic material (fibrin) and erythrocytes (hemorrhage). Foci measure up to 1.2 mm in diameter and are variably infiltrated by intact and degenerated neutrophils and fewer macrophages. Hepatocytes adjacent to the necrotic areas exhibit either hypereosinophilia with maintenance of cell border and karyorrhectic or pyknotic nuclei (coagulative necrosis). Other hepatocytes are swollen with pale eosinophilic and often finely vacuolated cytoplasm (degeneration). In some hepatocytes at the periphery of the necrosis numerous barely visible filamentous, pale basophilic bacilli (0.5 x 5 ?m) can be seen. Portal areas are moderately infiltrated by lymphocytes, plasma cells, macrophages and fewer neutrophils.
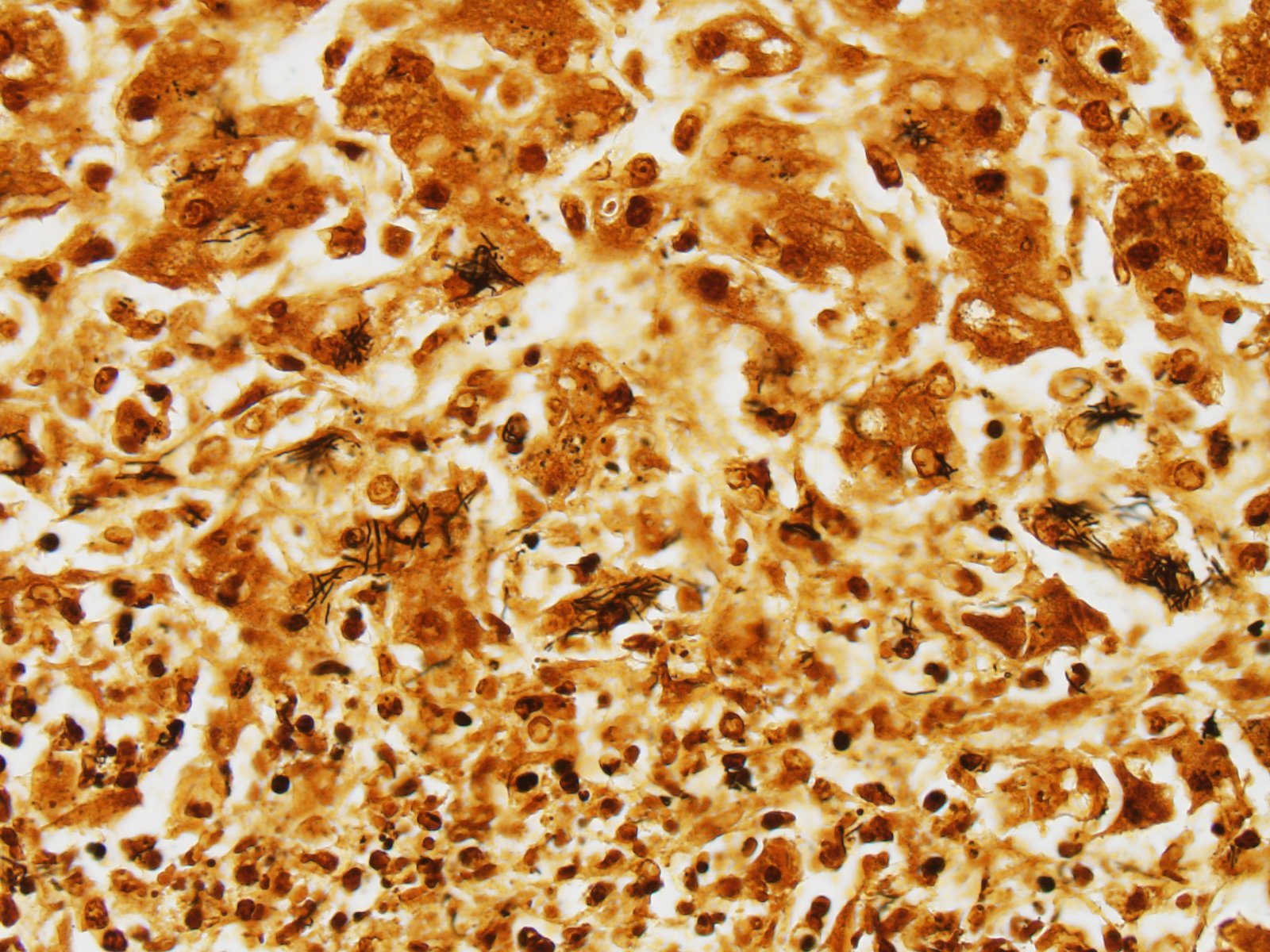
Using Warthin-Starry stain argyrophilic filamentous bacteria are visualized within hepatocytes at the periphery of necrotic areas and within the debris (Fig. 2).
Contributor’s Morphologic Diagnoses:
Liver: Hepatitis, random, necrotizing and suppurative, acute, multifocal to coalescing, severe with intracellular argyrophilic filamentous bacteria. Tyzzer´s disease.
Contributor’s Comment: A fatality in a young foal with a rapid course of disease, fever and jaundice, focused the postmortem examination on infectious diseases with highly virulent pathogens. Differentials after necropsy were infection with EHV-1 and bacterial sepsis (e.g. Enterobacteriaceae, Actinobacillus equuli). The typical histomorphology with random areas of necrosis and detection of argyrophilic bacteria were highly suggestive of Tyzzer´s disease. This was confirmed by PCR detecting a 633 bp fragment of Clostridium piliforme in a liver sample of this foal.
Clostridium piliforme, formerly known as Bacillus piliformis, is a gram-negative filamentous bacillus that is not cultivable in cell free media. In domestic animals, young foals are most often affected. Often, they are found dead or die after short illness.3 Wild rodents are believed to be the reservoir of the bacterium.
In 2013 Swerczek11 reviewed 148 cases of Tyzzer´s disease from central Kentucky and highlighted the predispositions, risk factors, and the mode of transmission. Foals in the first month of age are most frequently affected and at the highest risk for Tyzzer´s disease. Adult horses are believed to be resistant. There are more cases in spring in association with heavy rainfall. Interestingly, on 18th of March, two days before this foal came to the pasture, heavy rainfall started in the region of the herd in the western part of Germany (35 l/m2 rain in 48 hours). It is suggested that foals are infected by consuming feed contaminated with environmental spores of Clostridium piliforme or by ingestion of feces of the mares. Foals show coprophagy only from the second to the fifth week of life.10
Some cases of Tyzzer´s disease were reported in calves4 and in young dogs and cats, some of them suffering from immunosuppression.3,6,12
Mongolian gerbils are exquisitely susceptible to fatal Tyzzer´s disease, and many other laboratory animals (e.g. mice, rats, lagomorphs, hamsters, guinea pigs) are at risk for the infection with Clostridium piliforme.1 Cases were reported in marsupials2 and birds.5,7 The infection is extremely rare in nonhuman primates and humans.9 Recently, a case of Tyzzer´s disease was reported in a captive born cotton-top tamarin. A necrotizing typhlocolitis, accompanied by myocarditis was found additionally to the hepatic lesions .8
In the following weeks, no other cases of Tyzzer´s disease occurred in the herd of the affected foal.
We are grateful to Dr. W. Herbst, Institute of Hygiene and Infectious Diseases of Animals, University of Giessen, for performing the PCR.
Contributing Institution:
Institut fuer Veterinaer-Pathologie, Justus-Liebig-Universitaet Giessen
Frankfurter Str. 96, 35392 Giessen, Germany
http://www.uni-giessen.de/cms/fbz/fb10/institute_klinikum/institute/pathologie
JPC Diagnosis: Liver: Hepatitis, necrotizing, multifocal to coalescing, severe, with intracytoplasmic filamentous bacilli.
JPC Comment: The contributor has provided an excellent review of Tyzzer’s disease in a variety of species, and the previous case of Tyzzer’s disease in this year’s WSC Conference (Conf. 4, Case 1) in the colon of a cat should be reviewed for additional information on this often fatal disease.
In the foal, the disease is considered highly fatal but not contagious. Disease is seen between 1 and 6 weeks, with an average of 20 days.11 Outbreaks, first identified in Kentucky in 1964, may claim multiple animals on a farm, and are likely the result of soil contamination by these resistant bacilli and ingestion by foals. Another postulated route of infection is directly from the mare’s feces. It is thought that increased nutrition of nursing mares may result in a bloom of C. piliforme within the intestine. C. piliforme infection is extremely rare in adult healthy horse, but will result in an increased numbers within feces, and coprophagic foals may receive a large oral inoculum in this fashion.11
As opposed to the diseases in laboratory species, most cases of Tyzzer’s disease in the horse are primarily hepatic in nature. Enteric lesions may be present, but diarrhea is rare, and myocardial lesions are also very rare.
The moderator and attendees discussed the use of terms subacute and acute when describing this lesion. While a consensus was not reached, and both sides provided viable arguments, the moderator stressed the need for precision in the usage of such terms in reporting out equine cases, especially in insurance cases.
References:
- Barthold SW, Griffey SM, Percy DH. Pathology of laboratory rodents and rabbits, 4th Edition. John Wiley & Sons. 2016
- Canfield PJ, Hartley WJ. Tyzzer's disease (Bacillus piliformis) in Australian marsupials. J Comp Pathol. 1991;105(2):167-173.
- Cullen JM, Stalker MJ. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 6th ed. Vol 2. Elsevier, St. Louis, Missouri; 2015:258-352.
- Ikegami T, Shirota K, Une Y, Nomura Y, Wada Y, Goto K, et al. Naturally occurring Tyzzers disease in a calf. Vet Pathol. 1999;36:253-255.
- Mete A, Eigenheer A, Goodnight A, Woods L. Clostridium piliforme encephalitis in a weaver bird (Ploceus castaneiceps). J Vet Diagn Invest. 2011;23(6):1240-1242.
- Neto RT, Uzal FA, Hodzic E, Persiani M, Jolissaint S, Alcaraz A, Carvallo FR. Coinfection with Clostridium piliforme and Felid herpesvirus 1 in a kitten. J Vet Diagn Invest. 2015;27(4):547-551.
- Raymond JT, Topham K, Shirota K, Ikeda T, Garner MM. Tyzzer's disease in a neonatal rainbow lorikeet (Trichoglossus haematodus). Vet Pathol. 2001;38(3):326-327.
- Sasseville VG, Simon MA, Chalifoux LV, Lin KC, Mansfield KG. Naturally occurring Tyzzer's disease in cotton-top tamarins (Saguinus oedipus). Comp Med. 2007;57(1):125-127.
- Smith KJ, Skelton HG, Hilyard EJ, Hadfield T, Moeller RS, Tuur S, Decker C, Wagner KF, Angritt P. Bacillus piliformis infection (Tyzzer's disease) in a patient infected with HIV-1: confirmation with 16S ribosomal RNA sequence analysis. J Am Acad Dermatol. 1996;34(2 Pt 2):343-348.
- Soave O, Brand CD. Coprophagy in animals: a review. Cornell Vet. 1991;81(4):357-364.
- Swerczek TW. Tyzzer's disease in foals: retrospective studies from 1969 to 2010. Can Vet J. 2013;54(9):876-880.
- Young JK, Baker DC, Burney DP. Naturally occurring Tyzzers disease in a puppy. Vet Pathol. 1995;32:63-65.