CASE I: P20-1942 (JPC 4166940)
Signalment:
A four-year-old neutered male domestic cat (felis catus)
History:
Hyporexia and respiratory distress of approximately 5 days. A presumptive diagnosis of cardiac failure in emergency room. Bloodwork revealed azotemia and hyperglycemia although glucose dropped to normal over next 24h. After 4 days of supportive care, the owner selected euthanasia. This cat was perfectly healthy previous to this episode.
Gross Pathology:
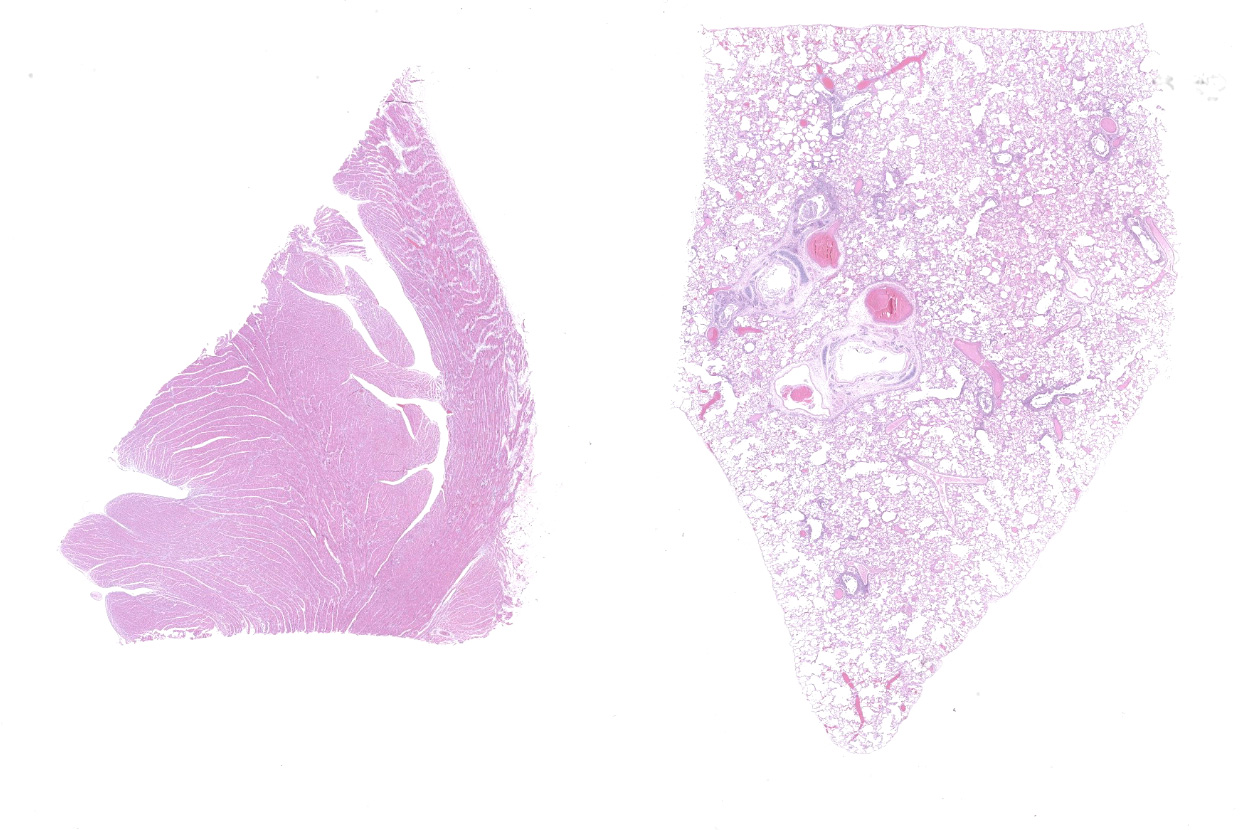
The ocular and gingival mucosa were slightly pale. Abundant (15 mL) red tinged fluid were collected from the thorax. The lungs were diffusely mottled red and slightly firm. The heart weight was 22 g. The left ventricular wall was markedly thickened, with a very narrow ventricular cavity (interpreted as hypertrophic cardiomyopathy). No significant findings in the rest of abdominal organs.
Laboratory Results:
Aerobic culture of the lung: No bacteria isolated after 48 hours. Feline Coronavirus PCR was performed with formalin fixed paraffin embedded tissues, which was negative.
Swabs in PBS (nasal, tracheal, oropharyngeal and rectal swabs) and tissues (lung, heart) were submitted to the Animal Health Diagnostic Center at Cornell University and initially subjected to PCR testing or culture for common feline respiratory pathogens (Bordetella sp., Chlamydia felis, Mycoplasma cynos, Mycoplasma felis, Streptococcus equi. ssp zooepidemicus, Influenza virus, pneumovirus, feline calicivirus and feline herpesvirus). The nasal swab sample tested positive for M. felis (Ct = 32), while no other common feline pathogen was detected nor isolated.
The nasal, oropharyngeal, tracheal and rectal swabs were positive for SARS-CoV-2 when tested by real-time RT-PCR assay (rRT-PCR). This result was later confirmed by NVSL. Immunohistochemistry and in situ hybridization for SARS-CoV-2 staining revealed strong viral labeling/staining in bronchial epithelial cells, bronchiolar glands, bronchiolar epithelium and in few desquamated epithelial cells/macrophages in alveolar spaces.
Microscopic Description:
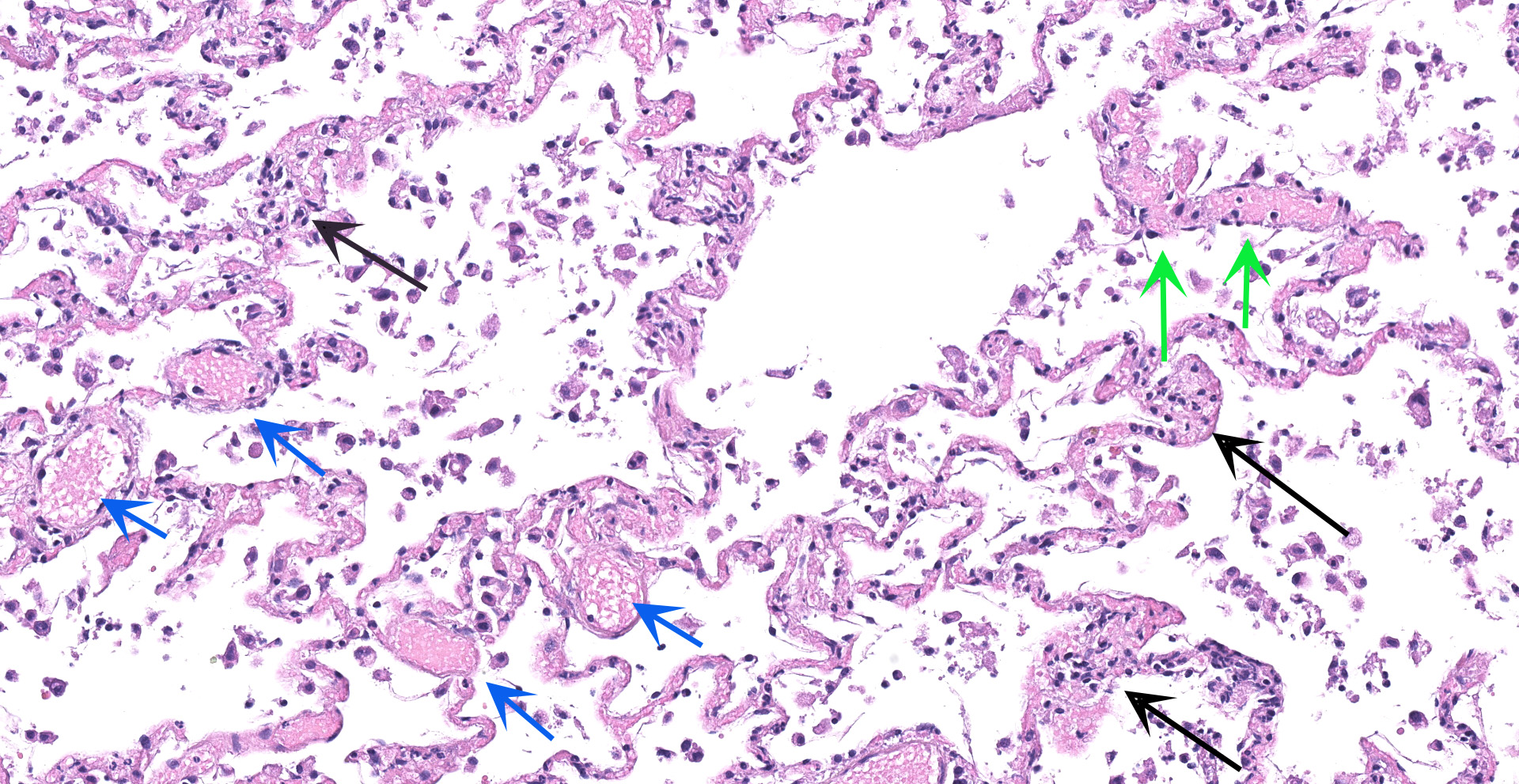
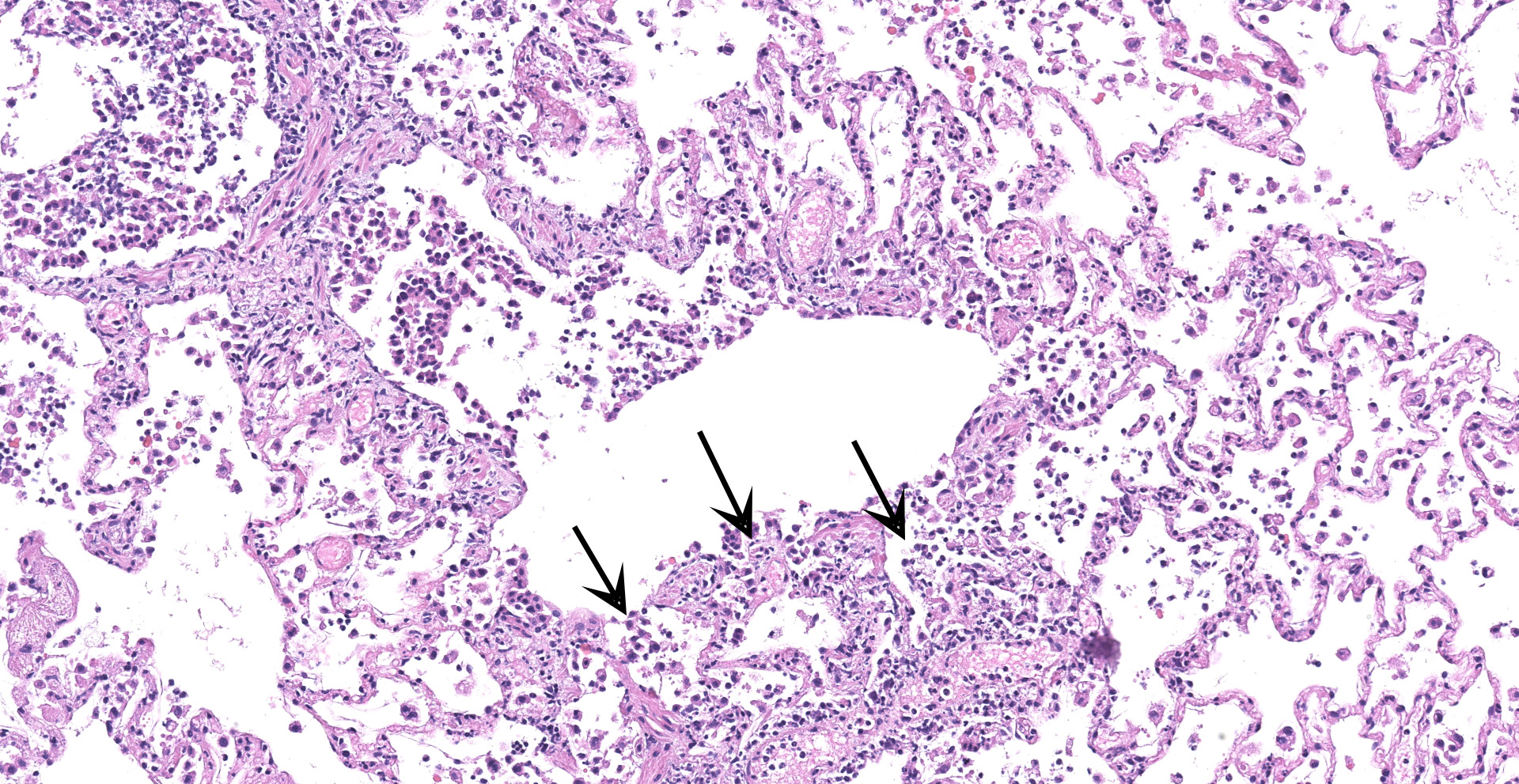
Lung: An acute bronchointerstitial pneumonia was noted. In bronchi, there was circumferential degeneration and necrosis of bronchial epithelial cells, with focal infiltrates of viable and degenerate neutrophils and macrophages. Similar inflammatory infiltrates were present surrounding bronchial glands. Necrosis of epithelial cells and similar inflammation was identified in the bronchiolar lumen, with some being completely occluded with cellular debris. In alveoli, damage was characterized by numerous macrophages with frequent phagocytosis of cellular debris, presence of multinucleated cells suggesting syncytial cells and a few neutrophils, all immersed in edema, multifocal hemorrhages, and fibrin, with the formation of rare hyaline membranes. There was multifocal mild segmental hyperplasia of type II pneumocytes. The alveolar septa were thickened with increased numbers of intravascular neutrophils and multiple segments of necrotizing capillaritis, with microthrombi and focal dense infiltrates of neutrophils and macrophages. Occasional arteries have transmural infiltrates of mononuclear cells with margination of neutrophils.
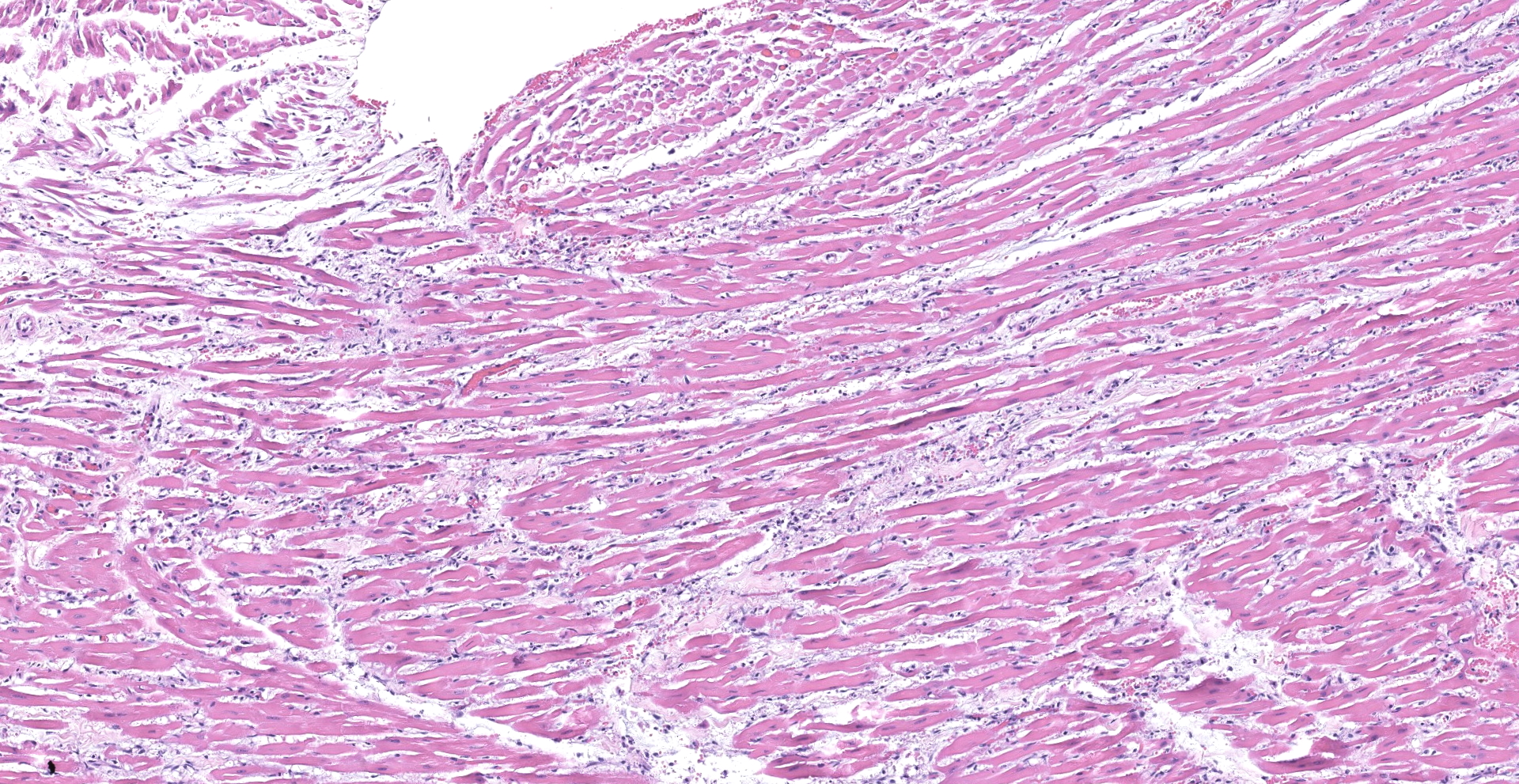
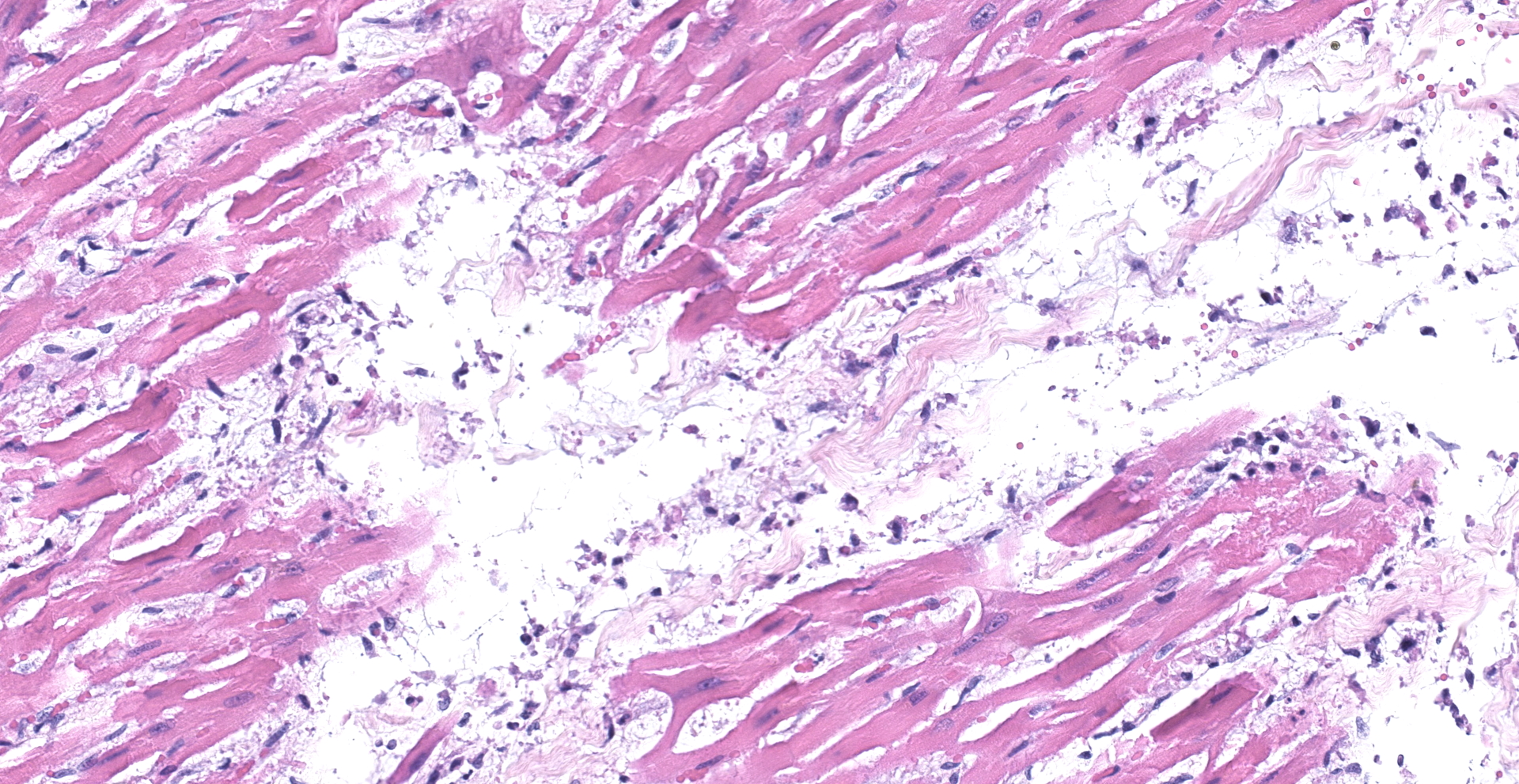
Heart: There was multifocal to coalescing areas of degeneration and necrosis of cardiomyocytes, with cells displaying hypereosinophilic cytoplasm, loss of striations, hyper contraction bands, vacuolation and or pyknotic nuclear debris. There was edema with the presence of small numbers of macrophages and neutrophils between myofibers In addition, there was mild concentric hypertrophy of the left ventricle. Cardiomyocytes have moderate variation of the cytoplasmic diameter, feature that was more frequent towards the periphery of the myocardium.
Contributor's Morphologic Diagnoses:
1. Lung: Pneumonia, bronchointerstitial, neutrophilic and histiocytic, acute to subacute, severe, with bronchial/bronchiolar necrosis, vascular thrombi and multifocal capillaritis.
2. Heart: Myocardial degeneration and necrosis, severe, multifocal to coalescing, with mild neutrophilic and histiocytic myocarditis.
Contributor's Comment:
Human to animal transmission and natural infections with SARS-CoV-2 have been reported in domestic cats, dogs, and ferrets as well as in wild animals in zoological collections around the world including tigers, lions and puma.13,14,16,20,23,24,25 Most SARS-CoV-2 infections in animals have been associated with subclinical- or only with mild clinical signs in affected animals.8,22,26
The predominant histologic pulmonary lesion in human patients that develop pneumonia due to the infection with SARS-CoV-2 is diffuse alveolar damage (DAD), including the exudative, proliferative and fibrotic phases or a mixture of these, with various levels of progression and severity. Changes include edema, congestion and formation of hyaline membranes, hyperplasia of atypical type II pneumocytes (reactive atypia) and increased numbers of macrophages and lesser neutrophils in the alveolar lumen; interstitial thickening with vascular thrombosis, CD4+ and CD8+ lymphocytes and macrophages infiltrates, endothelialitis and variable interstitial proliferation of myofibroblasts.1,2,4,6,7,10,11,12,18,21,28 Similar to the findings in humans, the histological changes observed in the lung of the cat in this study are indicative of DAD, with segmental proliferation of atypical type II pneumocytes (reactive atypia), thrombosis and focal areas of capillaritis and the inflammatory infiltrate being mainly composed of neutrophils and macrophages, which support an early disease stage. The infection was confirmed by IHC and ISH, suggesting direct viral damage to the lung.
The reported histological findings on the human heart in cases of severe SARS-C0V-2 infection are variable and range from no lesions, to a few interstitial mononuclear inflammatory infiltrates to myocardial degeneration and necrosis without significant inflammation.5,12,21,28 Histological examination of the heart of the cat in this case revealed acute myocardial degeneration and necrosis, with mild interstitial acute inflammation. These observations are compatible with an early manifestation of viral myocarditis but could also be secondary to a disseminated vascular damage.
One interesting finding of this case was the fact that the animal was affected by mild hypertrophic cardiomyopathy (HCM) with no previous clinical manifestations, as evidenced at necropsy by an enlarged ventricular wall and a narrow-left ventricle. The association of underlying cardiovascular disease and myocardial injury with severe and often fatal outcomes of COVID-19 in human patients has been well documented.15 It has been suggested that patients with hypertrophic cardiomyopathy have an increased ACE2 gene expression in the heart, which could be associated with an increased risk for severe COVID-19 manifestations in HCM patients.3,29 It is not known if cats with HCM have increased expression of the ACE2 gene. However, the fact that HCM has been observed as a comorbidity in other cats diagnosed with SARS-CoV-2 and that presented severe progressive respiratory disease,24 support the hypothesis that this common heart condition in cats could indeed predispose severe disease and poor prognosis in domestic cats that become infected with SARS-CoV-2.
Contributing Institution:
Virginia Maryland College of Veterinary Medicine.
205 Duck Pond Dr
Blacksburg, VA 24061
https://vetmed.vt.edu/departments/biomedical-sciences-and-pathobiology.html
JPC Diagnosis:
1. Lung: Pneumonia, bronchointerstitial, necrotizing and neutrophilic, diffuse, moderate, with multifocal septal necrosis and thrombosis.
2. Heart: Myofiber degeneration, necrosis, and loss, multifocal, with mild to moderate histiocytic and neutrophilic myocarditis, and edema.
JPC Comment:
The contributor provides a concise comparison of histological features characteristic of severe acute respiratory syndrome virus (SARS-CoV-2) infection in both humans and domestic felines.
SARS-CoV-2 is the etiologic agent of Coronavirus Disease 2019 (COVID-19). First identified in Wuhan City, Hubei Province, China in December 2019, COVID-19 quickly evolved into the ongoing and continually evolving global pandemic.17
SARS-CoV-2 is one of several species of the Betacoronavirus (βCoV) genus, which falls under the Coronaviridae and Orthocoronavirinae family and subfamily, respectively. In addition to βCoV, the Orthocoronavirinae subfamily includes genera Alphacoronavirus (αCoV), Deltacoronavirus (δCoV), and Gammacoronavirus (γCoV). These enveloped viruses contain a single-strand, positive-sense RNA genome, are 80-220nm in diameter, and infect a wide spectrum of wild and domestic species. In addition, a characteristic feature shared by these viruses is a unique "crown-like" appearance (corona: Latin for "crown") when viewed via electron microscopy due to the presence of superficial spike proteins.17
Avian species are primarily infected by δCoVs and γCoVs (e.g. avian CoV ? infectious bronchitis virus) with some spillover into mammals; αCoVs and βCoVs are restricted to mammals. Examples of αCoVs include feline infectious peritonitis virus and transmissible gastroenteritis virus in swine. Examples of βCoVs include bovine CoV and sialodacryoadenitis virus of rats in addition the highly pathogenic human βCoVs, which include severe acute respiratory syndrome (SARS) CoV-1, Middle East respiratory syndrome (MERS) CoV, and SARS-CoV-2.17
Coronaviruses are prone to mutation and recombination, greatly facilitating their propensity to not only undergo cross-species transmission, but also adapt and replicate in new host species following a spillover event. Examples of spillover events in veterinary species include the emergence of bovine CoV from equine CoV in the 1700s and more recently with both porcine epidemic diarrhea virus and swine acute diarrhea syndrome virus originating from bat strains. Notably, both SARS-CoV-1 and MERS CoV also originated from bat strains and infected intermediate hosts, civit cats and dromedary camels, respectively, prior to infecting humans. SARS-CoV-2 is suspected to have also undergone a similar host switching process, although the intermediate host is uncertain. Unfortunately, future spillover events into economically important livestock species and the human population are highly likely, particularly given the history of coronaviruses exhibiting a panache for interspecies transmission.17
As noted by the contributor, human to animal (i.e. reverse zoonosis) transmission of SARS-CoV-2 has been reported in multiple species, including domestic canines and felines, mink, and large cats in zoo collections.27 In addition, research models utilized include both old and new world non-human primates, mice, ferrets, cats, and hamsters. The majority of animal models utilized for research and development of SARS-CoV-2 prophylactics and therapeutics are nonhuman primates, Syrian hamsters, and mice.20
Research in regard to the pathogenesis of SARS-CoV-2 is ongoing. However, a key feature of the virus is its specific targeting of the angiotensin converting enzyme-2 (ACE2) receptor on host cell membranes. Multiple cell types express ACE2 receptors, including type I & II pneumocytes, endothelial cells, myocardiocytes, cholangiocytes, enterocytes, and oral mucosal epithelium.9 Once bound by the spike protein, proteases such as transmembrane serine protease 2, cathepsin B or L, or furin cleave the spike protein, leading to fusion with the host cell's membrane, infection, and ensuing cytopathic effects. Systemic disturbances may also occur, such as dysregulation of blood pressure due to downregulation of ACE2 receptors.17 Furthermore, it has been hypothesized human patients with certain underlying conditions, such as hypertension or COPD, are at increased risk due to upregulation of ACE2 receptors.21
In addition to respiratory disease, SARS-CoV-2 infection has also been reported to cause encephalitis, cerebral microhemorrhages, stroke, and diffuse leukoencephalopathy in humans. These lesions are hypothesized to occur due to viral binding to ACE2 rich endothelial cells in the CNS. In addition, both glial cells and neurons are reported to express ACE2 and may be targets of infection.9
The moderators discussed the importance of identifying and developing animal models that recapitulate lesions found in humans to allow effective and ethical investigation human diseases, such as with SARS-CoV-2. As noted by the contributor, most SARS-CoV-2 infections in humans are relatively mild, however severe disease tends to be associated with comorbidities, such as hypertension. Therefore it is important to identify animal models that not only represent the human population as a whole but also smaller subsets that may help identify "at risk" groups while also developing effective countermeasures applicable to those populations.
Conference participants also reviewed additional histochemical stains from this case, with Masson's trichrome revealing alveolar septa are mildly expanded by fibrosis. Participants hypothesized this case may have initially manifested as a subclinical chronic infection that was masked given typical feline behavior, followed by decompensation as the patient became hypoxic and ultimately presented in respiratory distress.
References:
1. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis and angiogenesis in COVID-19. N Engl J Med. 2020; 383: 120-128.
2. Borczuk AC, Salvatore SP, Seshan SV, et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Modern Pathol. 2020; 33: 2156-2168.
3. Bos JM, Hebl VB, Oberg AL, et al. Marked Up-Regulation of ACE2 in Hearts of Patients With Obstructive Hypertrophic Cardiomyopathy: Implications for SARS-CoV-2?Mediated COVID-19. Mayo Clin Proc 2020; 95, 1354?1368
4. Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS-CoV-2: targeting endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The mount Sinai COVID-19 autopsy experience. medRxiv 2020.05.18.20099960
5. Buja LM, Wolf DA, Zhao B, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020; 48: 107233.
6. Calabrese F, Pezzutto F, Fortarezza F, et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European pulmonary pathologists. Virchows Archiv 2020; 477: 359-372.
7. Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 2020; 20: 1135-1140.
8. Chandrashekar A, Liu J, Martino AJ, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020; 369: 812-817.
9. Choudhary S, Kanevsky I, Yildiz S, et al. Modeling SARS-CoV-2: Comparative Pathology in Rhesus Macaque and Golden Syrian Hamster Models [published online ahead of print, 2022 Feb 5]. Toxicol Pathol. 2022;1926233211072767.
10. Damiani S, Fiorentino M, De Palma A, et al. Pathological post-mortem findings in lungs infected with SARS-CoV-2. J Pathol 2021; 263: 31-40.
11. Edler C, Schröder AS, Aepfelbacher M, et al. Dying with SARS-CoV-2infection ? an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med 2020, 134: 1275-1284.
12. Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Res Med 2020; 8: 681-686.
13. Gaudreault NN, Trujillo JD, Carossino M, et al. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg Microbes Infect. 2020; 9: 2322-2332.
14. Gortázar C, Barroso-Arévalo S, Ferreras-Colino E, et al. Natural SARS-CoV-2 infection in kept ferrets, Spain. Emerg Infect Dis. 2021: 27: 1994-1996.
15. Guo T, Fan Y, Chen M, et al, Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020; 5: 811-818.
16. Halfmann PJ, Hatta M, Chiba S, et al. Transmission of SARS-CoV-2 in domestic cats. N Engl J Med 2020; 383; 592-594.
17. Kenney SP, Wang Q, Vlasova A, Jung K, Saif L. Naturally Occurring Animal Coronaviruses as Models for Studying Highly Pathogenic Human Coronaviral Disease. Vet Pathol. 2021;58(3):438-452.
18. Keresztesis AA, Perde F, Ghita-Nanu A, et al. Post mortem diagnosis and autopsy findings in SARS-CoV-2 infection. Forensic case series. Diagnostics 2020; 10: 1070.
19. Kumar A, Prasoon P, Kumari C, et al. SARS-CoV-2-specific virulence factors in COVID-19. J Med Virol. 2021;93(3):1343-1350.
20. McAloose D, Laverack M, Wang L, et al. From people to panthera: Natural sars-cov-2 infection in tigers and lions at the bronx zoo. MBio. 2020; 11: 1?13.
21. Martines RB, Ritter JM, Matkovic E, et al. Pathology and pathogenesis of SARS-CoV-2 associated with fatal Coronavirus disease, United States. Emerg Infect Dis 2020; 26: 2005-2015.
22. Munster VJ, Feldmann F, Williamson BN, et al. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020; 585: 268?272.
23. Patterson EI, Elia G, Grassi A, et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nat. Commun. 2020; 11: 1?5.
24. Segalés J, Puig M, Rodon J, et al. Detection of SARS-CoV-2 in a cat owned by a COVID-19-affected patient in Spain. PNAS 2020; 117: 24790-24793.
25. Sit THC, Brackman CJ, Ip SM, et al. Infection of dogs with SARS-CoV-2. Nature 2020; 586: 776-778.
26. Shi J, Wen Z, Zhong G, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 2020; 36: 1016?1020
27. Sooksawasdi Na Ayudhya S, Kuiken T. Reverse Zoonosis of COVID-19: Lessons From the 2009 Influenza Pandemic. Vet Pathol. 2021;58(2):234-242.
28. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8: 420-422.
29. Zhang H, Penniger JM, Li Y, et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020; 46: 586-590.
30. Patania OM, Chiba S, Halfmann PJ, et al. Pulmonary lesions induced by SARS-CoV-2 infection in domestic cats [published online ahead of print, 2021 Dec 29]. Vet Pathol. 2021;3009858211066840.
31. Rotstein DS, Peloquin S, Proia K, et al. Investigation of SARS-CoV-2 infection and associated lesions in exotic and companion animals [published online ahead of print, 2022 Jan 18]. Vet Pathol. 2022;3009858211067467.
32. Choudhary S, Kanevsky I, Yildiz S, et al. Modeling SARS-CoV-2: Comparative Pathology in Rhesus Macaque and Golden Syrian Hamster Models [published online ahead of print, 2022 Feb 5].