Signalment:
4-year-old, male castrated, Italian greyhound breed canine (
Canis familiaris)This dog presented to the critical care service
with a primary complaint from the referring veterinarian
of possible toxicity and/or multi-organ failure. The owner
reported that the night before presentation, the dog had
vomited 18 times, and that since then the dog was listless
and weak, and had not urinated since the onset of clinical
signs. The dog was housed with three other dogs, including
one who exhibited vomiting and diarrhea three days prior
to this dogs illness but had recovered uneventfully.
Gross Description:
Autolysis was moderate. The right
and left kidneys were diffusely purple-black, and the urinary
bladder contained approximately 1 ml of dark red urine.
Petechial hemorrhages were present on visceral pleural
surfaces of all lung lobes, and there was mild pulmonary
edema. The peritoneal cavity contained approximately 150
ml of serosanguineous fluid. The entire gastrointestinal tract
from stomach to rectum contained scant, tenacious, brownblack
material resembling digested blood. The spleen
was contracted, with an absence of blood in the red pulp.
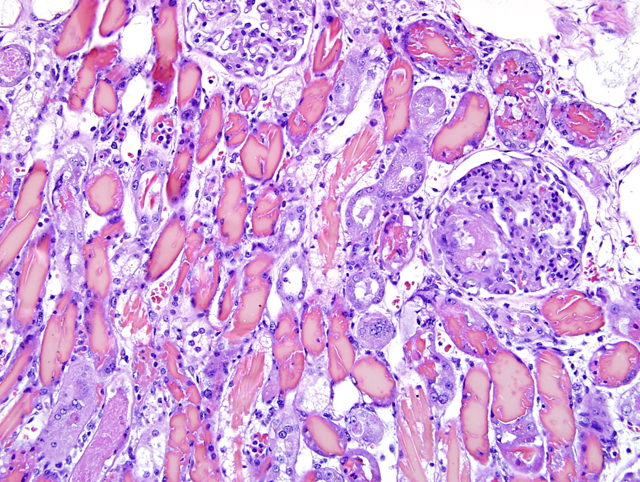
Histopathologic Description:
Diffusely, most
cortical distal tubules, collecting ducts, and medullary
tubules contain extensive intraluminal casts (
Fig. 1-1).
Fewer casts are present in cortical proximal tubules.
The majority of casts consist of amorphous to elongate
crystalline, brightly eosinophilic material (hemoglobin
casts) with fewer casts composed of eosinophilic,
amorphous, globular or granular material variably
admixed with sloughed epithelial cells (hyaline, cellular
or granular casts). Less severely affected tubules within
the cortex contain globular proteinaceous debris. When
viewed with polarized light, casts contain small numbers
of refractile, white, acicular, 5um x 20-30um crystals.
The epithelium lining these tubules is in various stages
of degeneration, necrosis, and early regeneration,
characterized by cells that are markedly attenuated,
hypereosinophilic with pyknotic or karyorrhectic nuclei,
or sloughed into the tubular lumen (degeneration and
necrosis). Some cells display anisocytosis, anisokaryosis,
aggregation, hyperchromatic nuclei and occasional
mitoses (regeneration). Scattered tubular epithelial cells
contain brown granular cytoplasmic pigment. The tubular
basement membrane is multifocally disrupted, and the
adjacent interstitium is mildly edematous and has a mild
interstitial infiltrate of neutrophils. Glomeruli diffusely
have moderate amounts of proteinaceous material within
Bowmans space. Within the vasa recta, there is variable
congestion and neutrophilic leukostasis.
Morphologic Diagnosis:
Tubular
epithelial degeneration and necrosis, acute, diffuse, severe,
with regeneration, and marked intratubular hemoglobin,
hyaline, and granular cast formation, consistent with
hemoglobinuric nephrosis, kidney, Italian greyhound
breed canine
Lab Results:
At presentation to the teaching
hospital the dogs hematocrit was 5% (37-55), white blood
cell count was 51.7 x103/ul (6-17) (95% neutrophils, no
bands), hemolysis index 2567 (3-56), creatinine was 6.9
mg/dl (0.6-1.4), and blood urea nitrogen was 185 mg/dl
(12-26). The dog was anuric, and no urine was available
for diagnostic testing. A Coombs test was not performed
on this patient.
Condition:
Hemoglobinuric nephrosis
Contributor Comment:
Clinical pathology
data for this patient suggests an acute intravascular
hemolysis, likely secondary to autoimmune mechanisms.
The proximal tubular necrosis, in conjunction with
the dramatic, widespread accumulation of obstructive
hemoglobin casts, is consistent with peracute primary
hemoglobin toxicity. Primary hemoglobin nephrotoxicity
has a controversial role in veterinary medicine, although
it is widely recognized in human medicine as a common
and serious postoperative complication following
cardiopulmonary bypass
1 and, less commonly, in
certain envenomations. Two diverging theories hold
that hemoglobin is 1) not a direct nephrotoxin, but rather
induces renal damage by a combination of hemoglobininduced
hypotension, increased vascular resistance, and
disseminated intravascular coagulation resulting in renal
ischemia; or 2) that hemoglobin is not directly toxic, but
is converted to toxic byproducts within the urinary space,
thereby inducing tubular necrosis.
3 In experimental studies
utilizing intravenously administered purified hemoglobin
in rats, Zager et. al.Â
3concluded that hemoglobin can act as
a primary nephrotoxin only in the presence of aciduria, and
that the likely mechanism for this is aciduria-dependent
conversion of hemoglobin to methemoglobin, the latter of
which precipitates within distal tubule segments and forms
casts. Unlike hemoglobin, once methemoglobin forms its
precipitation can occur under either aciduric or alkalinuric
conditions, and is more toxic than hemoglobin under
either condition. Furthermore, distal tubular obstruction
can lead to enhanced upstream uptake of hemoglobin
and its metabolites by proximal tubules.
3 Tubular damage
in this case likely resulted from both local and systemic
contributing factors, including massive, widespread
tubular obstruction and attendant tubular epithelial
necrosis, hypoxia secondary to the markedly decreased
hematocrit, and hemoglobin-induced vasoconstriction
via activation of the renin-angiotensin and sympathetic
nervous system.
2 The patient had mildly to moderately elevated D-dimers
and partial thromboplastin time, but normal prothrombin
time and no clinical evidence of bleeding. Calculation of
the lipemia, free hemoglobin, and bilirubin is performed
on a clinical chemistry analyzer by passing light of
different wavelengths through the serum or plasma and
calculating the amount of each interfering substance based
on the light absorption of the sample. The amount of free
hemoglobin is reported as the hemolysis index in units of
mg/dL. Elevations as high as those present in this case can
falsely decrease bicarbonate, GGT, amylase, and alkaline
phosphatase, and can falsely increase AST, CK, iron,
phosphate, triglycerides, magnesium, and total protein.
JPC Diagnosis:
Kidney: Tubular degeneration,
necrosis, and regeneration, diffuse, marked with
hemoglobin and granular casts and rare glomerular fibrin
thrombi
Conference Comment:
Acute renal failure is often
caused by acute tubular necrosis. Acute tubular necrosis
is usually caused by either nephrotoxic agents from the
bloodstream, ischemia, or complete urinary outflow
obstruction. The anatomic locations most susceptible to
acute tubular necrosis are the proximal convoluted tubule
and the thick ascending limb of the loop of Henle. This
is in direct correlation to their high metabolic activity thus
making them more susceptible to damage and necrosis.
2
Ischemic tubular necrosis usually follows profound
shock, and the extreme decrease in blood flow to the
kidney results in renal cortical necrosis. The histologic
hallmark of ischemic acute tubular necrosis is necrosis of
the proximal, and to a lesser extent, distal tubules with
disruption of tubular basement membranes and plugging
of tubular lumina by casts. If the basement membrane is
extensively damaged, then regeneration is impossible and
the prognosis becomes grave.
2
Nephrotoxic acute tubular necrosis causes necrosis of
the proximal convoluted tubule because of the PCTs
exposure to more toxin than the rest of the nephron as well
as its high metabolic activity. The basement membrane is
normally preserved in cases of nephrotoxic acute tubular
necrosis providing scaffolding for cells to regenerate thus
making the prognosis much more favorable than ischemic
acute tubular necrosis.
2
During the conference, several causes of tubular necrosis
were discussed. Aminoglycosides, tetracyclines,
sulfonamides, and the antifungal agent amphotericin B can
cause tubular necrosis in domestic animals.
2 Ethylene
glycol is also a common cause of tubular necrosis in dogs
and cats. Oxalate containing plants including
Halgeton
glomeratus (halogeton),
Sarcobatus vermiculatas
(greasewood),
Rheum rhaponticum (rhubarb) are a few
of the oxalate containing plants that cause poisoning
in sheep and cattle.
2 Other plants that cause tubular
necrosis include Easter lily in cats,
Quercus spp. (oak) in
ruminants, and
Amaranthus retroflexus (pigweed) in pigs
and cattle.
2Apergillus niger and
flavus can also produce
enough oxalates to cause renal damage when ingested with
feedstuffs.
2 Grapes and raisins cause tubular necrosis in
dogs.
2
References:
1. Haase M, Haase-Fielitz A, Bagshaw SM, Ronco C,
Bellomo R: Cardiopulmonary bypass-associated acute
kidney injury: a pigment nephropathy? Contrib Nephrol
156:340-353, 2007
2. Schlafer DH, Miller, RB: Inflammatory Diseases of
the Uterus.Â
In: Jubb, Kennedy, and Palmers Pathology
of Domestic Animals, ed. Maxie, MG, 5th ed., vol. 2, pp
466-469. Elsevier, Philadelphia, USA, 2007
3. Zager RA, Gamelin LM: Pathogenetic mechanisms in
experimental hemoglobinuric acute renal failure. Am J
Physiol
256:F446-F455, 1989