Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 9
1 November 2018
CASE IV: 13101599 (JPC 4048573-00).
Signalment: 13-month-old, intact male, Angus, Bos taurus, bovine
History: Two, 13-month-old, Angus bulls from the same farm presented for acute, voluminous, red, watery diarrhea. The diarrhea was noticed by the owner while animals were being gathered into the barn for a routine testing procedure. Both bulls were in excellent body condition (body condition score of 5/9) and one of the two was said to be the owners fastest grower. Both bulls were routinely vaccinated and dewormed. One of the bulls died while in transit to Oklahoma State University and a necropsy was performed at the Oklahoma Animal Disease Diagnostic Laboratory. Multiple diagnostic tests were performed on the other bull and the only significant finding on rectal examination was frank blood within the colon and a CBC measuring a PCV of 19%. This bull fully recovered with symptomatic care.
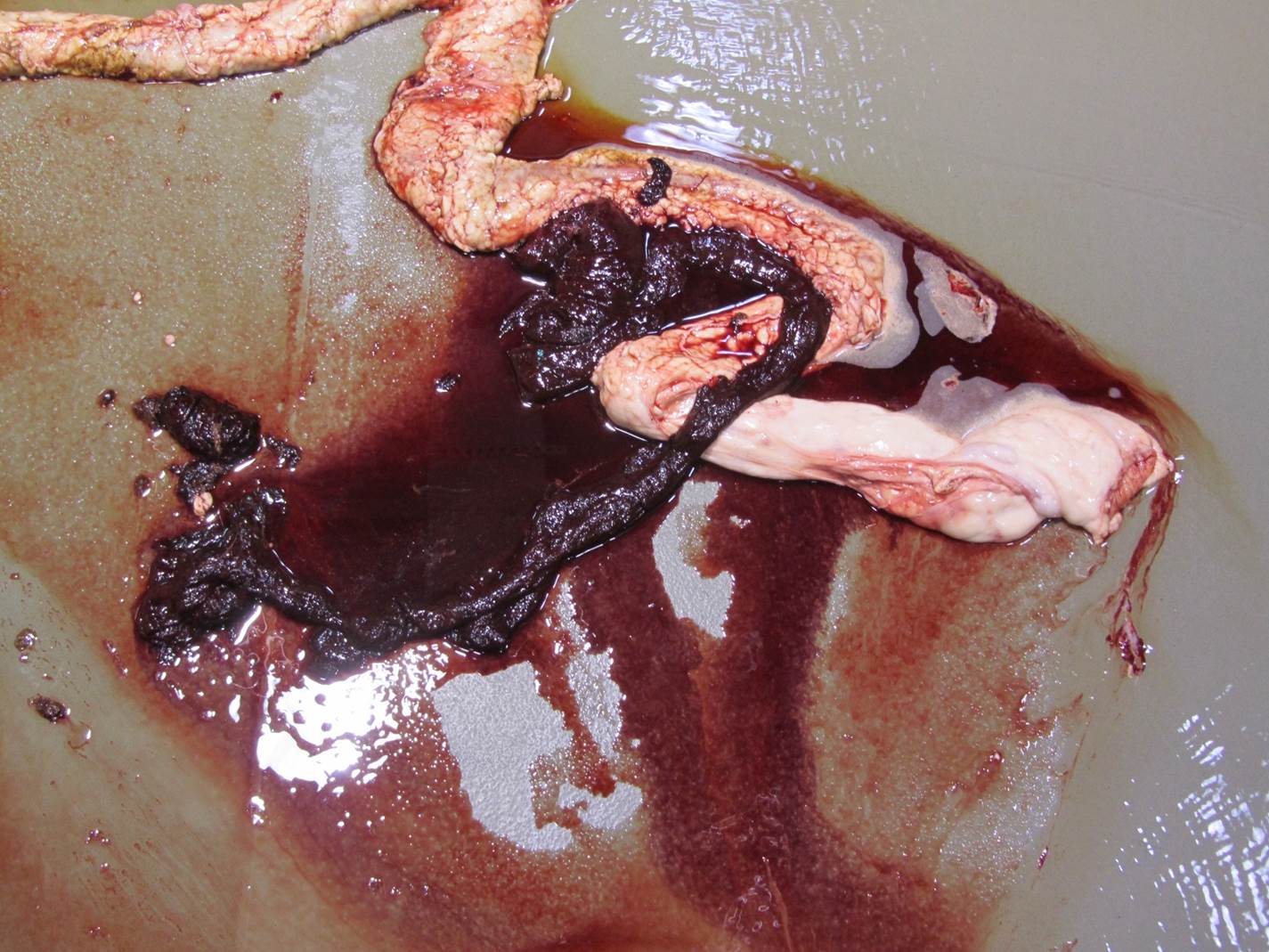
Gross Pathology: On gross examination, the bull was in excellent body condition. Approximately 7cm proximal to the spiral colon, there was an abrupt change in the intestinal content from normal to watery dark red fluid containing blood clots. This continued throughout the remainder of the spiral and descending colon. Segmentally (30 cm), the distal colonic mucosa had a mild cobblestone appearance with innumerable pinpoint to coalescing petechiae and ecchymosis and approximately four 0.5 x 0.2 cm, elliptical, shallow mucosal ulcers. Otherwise, the colonic mucosa outside of this segment was within normal limits.
Laboratory results:
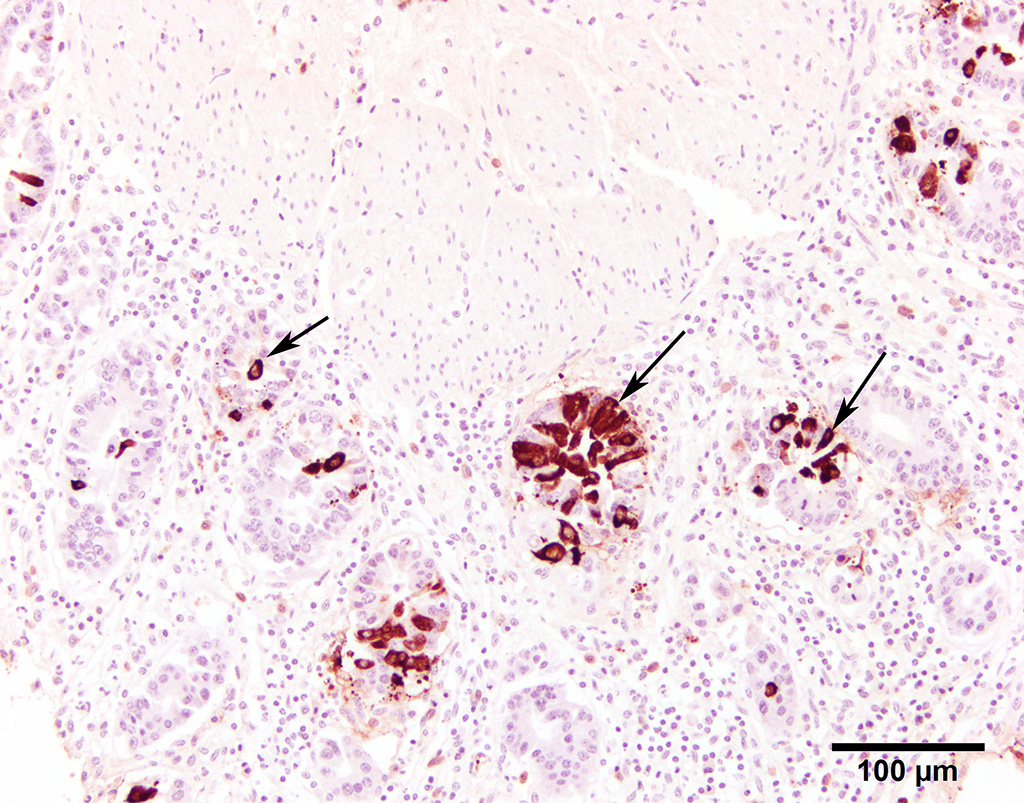
Fluorescent antibody (FA) staining for coronaviral antigens was conspicuously positive in the colonic mucosal sections. Immunostains (IHC) were also strongly positive (13101599 BCoV IHC). Positive and negative controls for both FA and IHC stains behaved as expected.
Bacterial culture of distal colon, spiral colon, and feces yielded large numbers of growth of E. coli and Streptococcus bovis. A Clostridium perfringens Type A was also isolated, but was determined opportunistic with little contributory role to death.
Fecal floatation was negative for parasites, including coccidia.
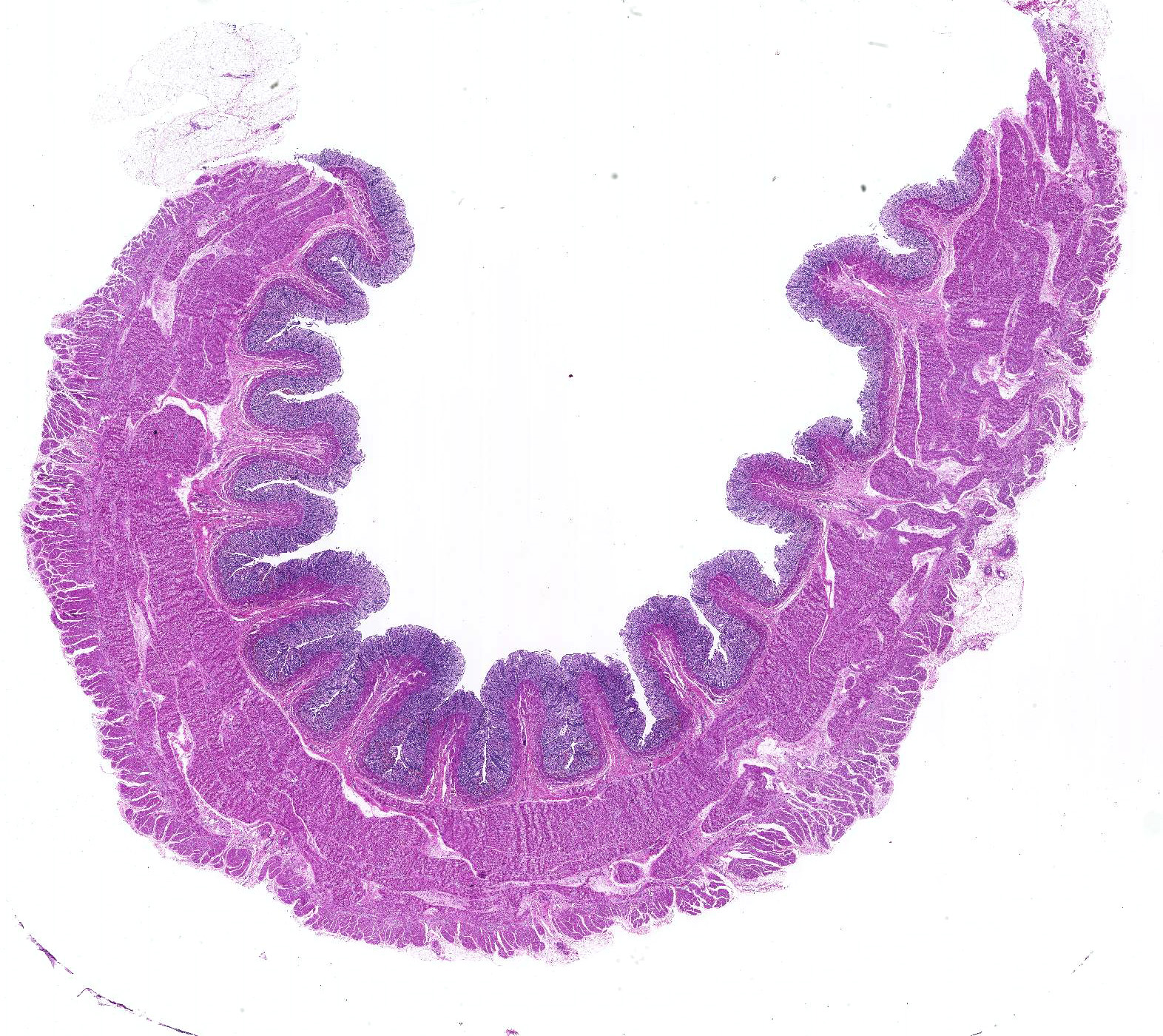
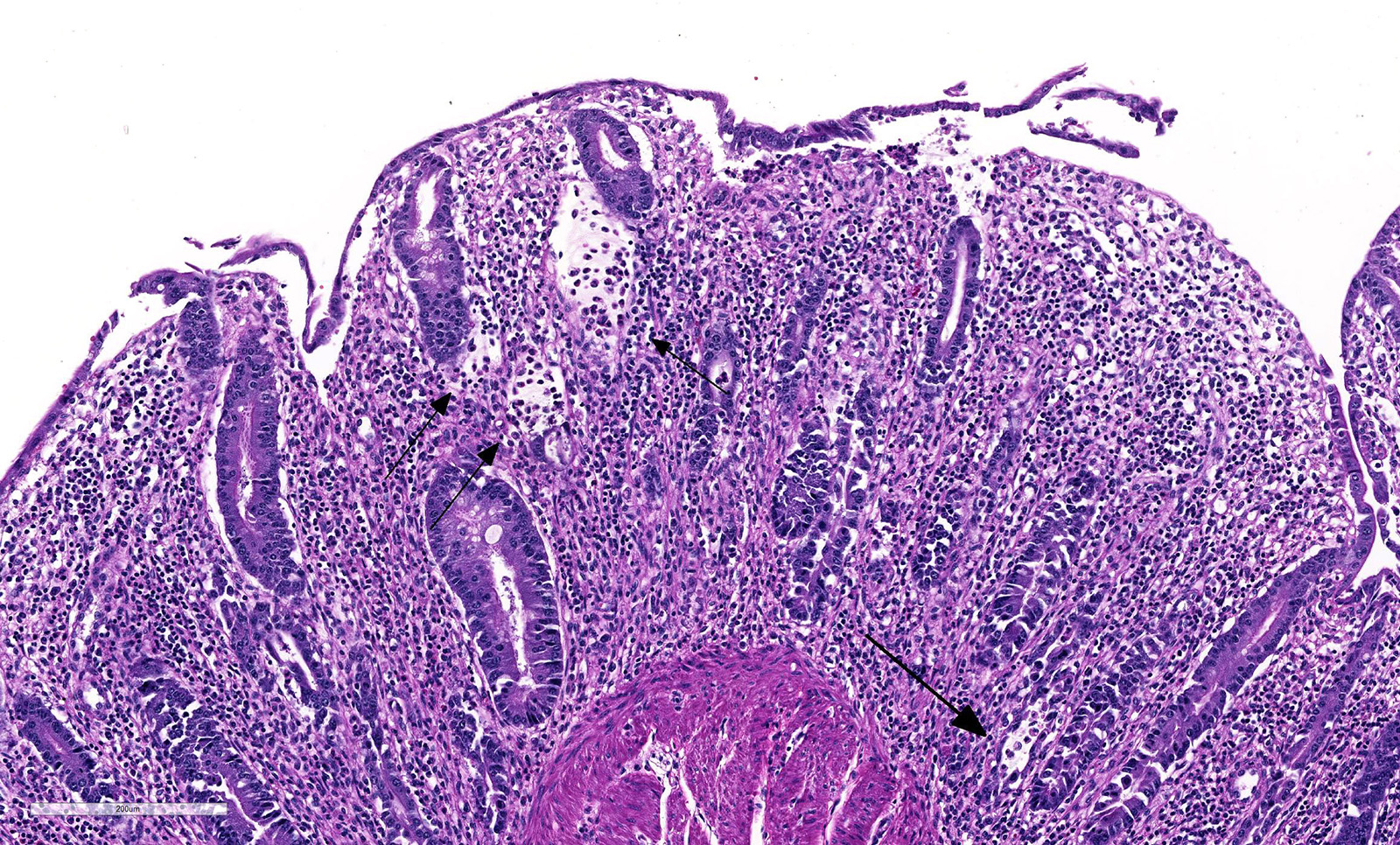
Microscopic Description: Histologically, colonic sections exhibited multifocal loss of colonic glands with scattered remnant glands present in an uneven distribution. The remnant glandular epithelium was either hyperplastic or was exhibiting features of degeneration and necrosis with occasional sloughing of necrotic epithelial cells into the glandular lumen. The hyperplastic epithelium exhibited re-epithelialization of expansive sections of the surface mucosa. In most regions, goblet cells were absent. The lamina propria had a moderate infiltrate of lymphocytes and plasma cells. Multifocally, hemorrhage was present within the lamina propria.
Contributor’s Morphologic Diagnoses:
Large intestine, distal colon: Ulcerative colitis, lymphoplasmacytic and hemorrhagic with multifocal colonic epithelial necrosis and colonic gland collapse, severe
Contributor’s Comment: Coronavirus has been implemented in causing three diseases in cattle: neonatal diarrhea in calves (between 1 day and 30 months old, with diarrhea most common at 1 to 2 weeks of age), respiratory disease in a wide age range of calves (between 2 and 19 weeks of age), and winter dysentery, which affects adult dairy and beef cattle.1
Genus Coronavirus is found in the family Coronaviridae within the genus Torovirus. They have a single-stranded, positive sense, RNA genome, are pleomorphic and vary in size from 70 to 200 nm in diameter.2 They contain four major structural proteins: the nucleocapsid protein N, the integral membrane glycoprotein M, the spike glycoprotein S, and the hemagglutinin-esterase glycoprotein HE. N protein is associated with the genome to form the nucleocapsid. M glycoproteins are found within the virus envelope. S and HE glycoprotein receptors are used in attachment of the virus to the target cell membrane.1
The exact cause of winter dysentery has not yet been determined; however mounting scientific evidence strongly suggests the etiologic agent to be coronavirus. Winter dysentery has been described in cattle worldwide, and in the United States, is more common in the northern states.1,3 Typically, this disease occurs in cattle during the cooler winter months and is associated with multiple environmental risk factors, including low drop in atmospheric temperature, close confinement, poor ventilation, and using manure handling equipment to handle feed.3 Viral transmission is by respiratory or oral routes.
Winter dysentery is a sporadic, acute, contagious, hemorrhagic enterocolitis that predominately affects adult dairy cows, and infrequently adult beef cattle. Clinical signs include dark brown, hemorrhagic, watery, and commonly profuse diarrhea, often with concurrent anorexia, depression, and dehydration. The disease typically has a high morbidity (50-100%) and low mortality (1-2%). Significant economic losses are associated with this disease, especially regarding milk drops in lactating dairy cows.3 The length of acute disease is brief, often with spontaneous recovery within a few days. Persistent diarrhea can lead to dehydration, with secondary polydipsia and reduced ruminal motility. Rarely, hemorrhage from the large colon leads to acute, severe anemia and death4, as seen in this case.
Differential diagnoses for adult cattle with acute, profuse, watery, diarrhea include BVDV, coccidiosis, and salmonellosis. Diagnosis of winter dysentery requires physical examination and exclusion of the above causes of acute and contagious diarrhea in cattle. Diagnostic tests include PCR, virus isolation, or IHC on ear notch to rule out BVDV, fecal floatation to rule out coccidiosis, and fecal bacterial culture to rule out salmonellosis. At necropsy, spiral colon is the ideal sample for virus detection because the virus persists for the longest time here after oral infection.3 Immunohistochemistry was used in this case to detect BCoV antigen in positively staining crypts within the colonic mucosal epithelium.
Coronavirus has a tropism for respiratory and intestinal epithelium.4 Coronavirus implicated in winter dysentery causes lesions comparable to neonatal calves with coronaviral diarrhea. In winter dysentery, epithelial cells of colonic crypts are destroyed by virus, leading to degeneration and necrosis of crypt epithelium. Sloughing of damaged and necrotic epithelium can be seen grossly as massive quantities of frank, often clotted, blood within the spiral and distal colon. Viral particles leave the cell by exocytosis at the apical or lateral cell surface, or by lysis of the cell.2,3 As in this case, marked mucosal atrophy and re-epithelialization can also be seen. In addition, fine streaks of hemorrhage were noted along the edges of the mucosal folds in the distal colon. 2,3
The diarrhea that occurs in winter dysentery is a malabsorptive diarrhea and death in this case was most likely due to severe, acute anemia from profound colonic hemorrhages.
Contributing Institution:
Department of Veterinary Pathobiology
Center for Veterinary Health Sciences
Oklahoma State University
www.cvm.okstate.edu
JPC Diagnosis: Colon: Colitis, necroulcerative and hemorrhagic, diffuse, severe with marked loss of glands and multifocal crypt abscessation.
JPC Comment: The contributors provide an excellent review of this case, and subsequent to its submission for the Wednesday Slide Conference, published this is a case report including three additional cases, from which further details are now publicly available. In 2012-2013, four calves from two herds died of acute necrohemorrhagic colitis. Colonic tissue from all calves were positive by fluorescent antibody and IHC for BoCV. The virus was isolated, and the genomic information from a variable region of the Spike gene revealed BoCV clade 2 in all cases, which had been previously seen as a respiratory infection in postweaned beef calves. (To date, 4 separate clades of BoCV have been isolated.)
Coronaviruses are well known as pathogens of both the digestive system (dog, swine, mice, rats) as well as the respiratory system (humans, cattle, rats). In the early 2000s, SARS-CoVs was discovered in civet cats, raccoon dogs, and bats) that were associated with both respiratory and enteric infections, and were subsequently identified in humans, camelids, and a wide variety of species. BoCV in itself is a pneumoenteric virus infecting both the upper and lower respiratory tract in respiratory infections.
BoCV is ubiquitous worldwide based on antibody seroprevalence data In a study of 2311 calves with diarrhea in Tulare, CA, 30.5% were positive for BCV; the age range of affected animals was 1-30 days with an average of 10.4 days.1 BoCV is also commonly incriminated as one of the inciting agents in the bovine respiratory disease complex in feedlot cattle with some studies identifying the virus through the use of RT-PCR testing in up to 96% of feedlot cattle. A number of wild species may serve as wildlife reservoirs for BoCV, including white-tailed deer, Sambar deer, elk, waterbuck, and giraffes; isolates from each of these species share amino acid sequences with over 99% homology to BoCV. Dogs have also been implicated as a potential reservoir for a coronavirus which may potentially infect ruminants.
In 2-6 month old calves, respiratory infections are associated with coughing, rhinitis, and pneumonia, which may or may not be accompanied by enteric signs; fecal shedding of the virus is commonly documented. It has been proposed by researchers following the time course of pneumoenteric infections that following clinical upper respiratory infection, large amounts of the virus, coated in mucus, may be swallowed, facilitating infection of the intestinal epithelium. A number of intra-nasal modified live vaccines designed primarily to protect against coronavirus-associated “calf scours” have been released on the market in the last five years.
The moderator gave the following differentials – BoCV, BRotaV, Coccidiosis (gross appearance, but too old an animal), and salmonellosis. The moderator commented on the age of the affected (13 months) in this case, which is consistent with “winter dysentery” (which is another misnomer as it may occur in hot months or in tropical climates which do not see a cold season as well.)7
References:
- Blanchard PC. Diagnostics of dairy and beef cattle diarrhea. Vet Clin North Am Food Anim Pract. 2012 Nov;28(3):443-64. doi: 10.1016/j.cvfa.2012.07.002.
- Boileau M, Kapil S: Bovine coronavirus associated syndromes. Vet Clin North Am Food Anim Pract 26:123-146, 2010.
- Brown CC, Baker DC, Barker IK: Bovine Coronavirus. In: Alimentary system, in Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, M.G. Maxie, Editor. 2007, Elsevier Saunders: Philadelphia, PA. p. 172-173.
- Clark, MA: Bovine Coronavirus. Br Vet J 149:51-70,1993.
- Fulton RW, Herd HR, Sorensen NJ, Confer AW, Ritchey JW, Ridpath JF, Burge LJ. Enteric disase in postweaned beef calves associated with bovine coronavirus clade 2. J Vet Diagn Invest 2015; 27(1):97-101.
- Natsuaki S, Goto K, Nakamura K, Yamada M, Ueo H, Komori T, Shirakawa H, Uchinuno Y: Fatal Winter Dysentry with Severe Anemia in An Adult Cow. J Vet Med Sci 69:957-960, 2007.
- Uzal FA, Plattner BL, Hostetter JM. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier;2016:216-217.)
1. Boileau M, Kapil S: Bovine coronavirus associated syndromes. Vet Clin North Am Food Anim Pract 26:123-146, 2010.
- Brown CC, Baker DC, Barker IK: Bovine Coronavirus. In: Alimentary system, in Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, M.G. Maxie, Editor. 2007, Elsevier Saunders: Philadelphia, PA. p. 172-173.
- Clark, MA: Bovine Coronavirus: Br Vet J 149:51-70,1993.
- Fulton RW, Herd HR, Sorensen NJ, Confer AW, Ritchey JW, Ridpath JF, Burge LJ. Enteric disase in postweaned beef calves associated with bovine coronavirus clade 2. J Vet Diagn Invest 2015; 27(1):97-101.
- Natsuaki S, Goto K, Nakamura K, Yamada M, Ueo H, Komori T, Shirakawa H, Uchinuno Y: Fatal Winter Dysentry with Severe Anemia in An Adult Cow. J Vet Med Sci 69:957-960, 2007.
- Uzal FA, Plattner BL, Hostetter JM. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier;2016:216-217.)