CASE 1: 41375 (4117060-00)
Signalment:
7-year-old male (gelded) cob, equine (Equus caballus)
History:
The gelding presented with a 48-hour history of colic. Signs of abdominal pain (colic) and mild ptosis were evident on clinical examination. Nasogastric reflux was present on intubation. Rectal examination and abdominal ultrasound revealed distended and non-motile small intestinal loops, and non-compressible impaction of the colon. Medical management was initiated however, due to unrelenting abdominal pain, and the clinical suspicion of grass sickness, euthanasia was carried out.
Gross Pathology:
The horse was in good body condition. Gross findings were limited to the gastrointestinal tract. All 4 divisions of the ascending colon contain firmly compacted and dry content (impaction) that has a variably present black coating (most prominent in the more distal regions, especially the right dorsal colon). The small intestine was dilated by gas and a moderate amount of liquid.
Laboratory results:
Not supplied.
Microscopic description:
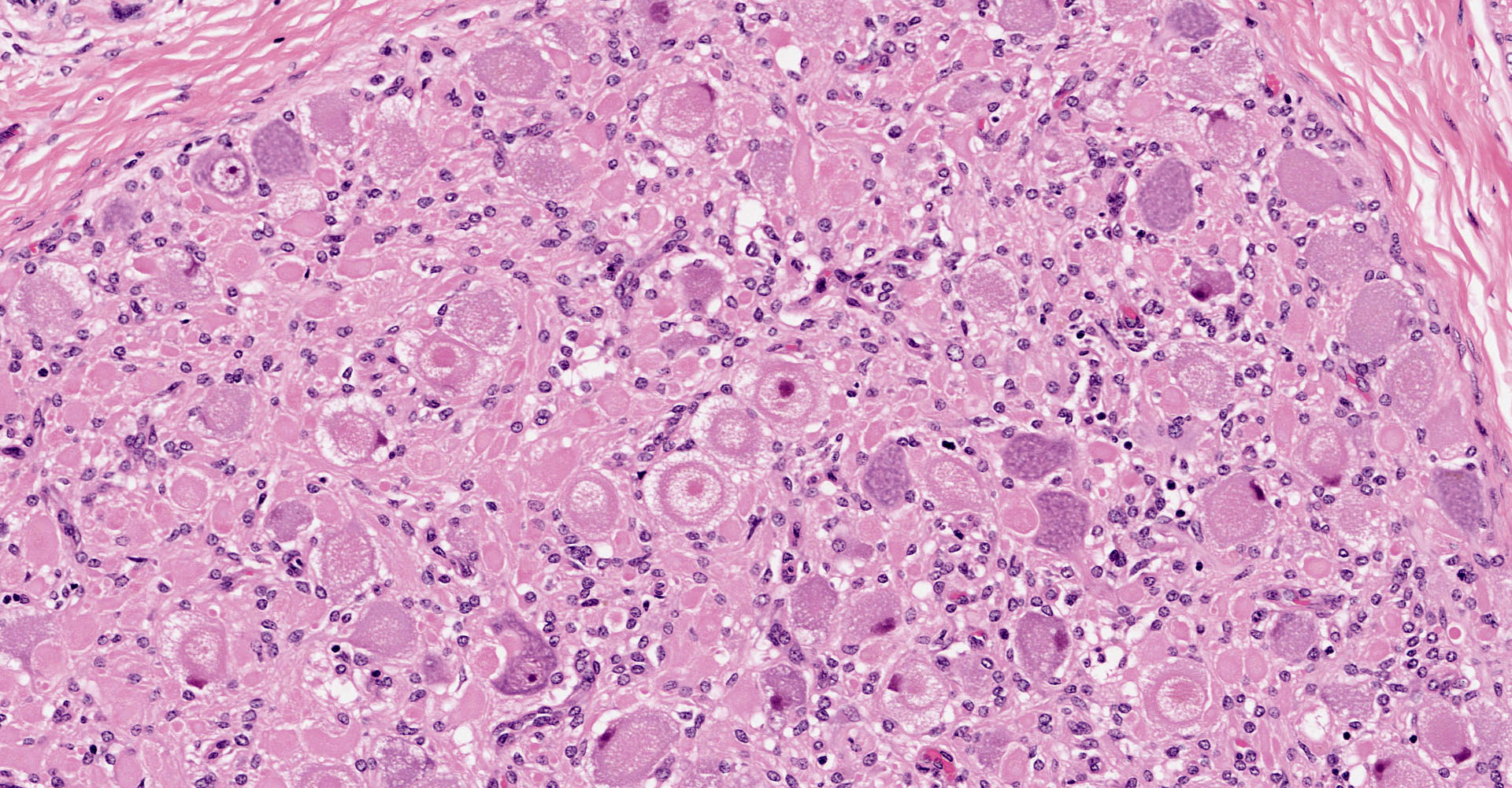
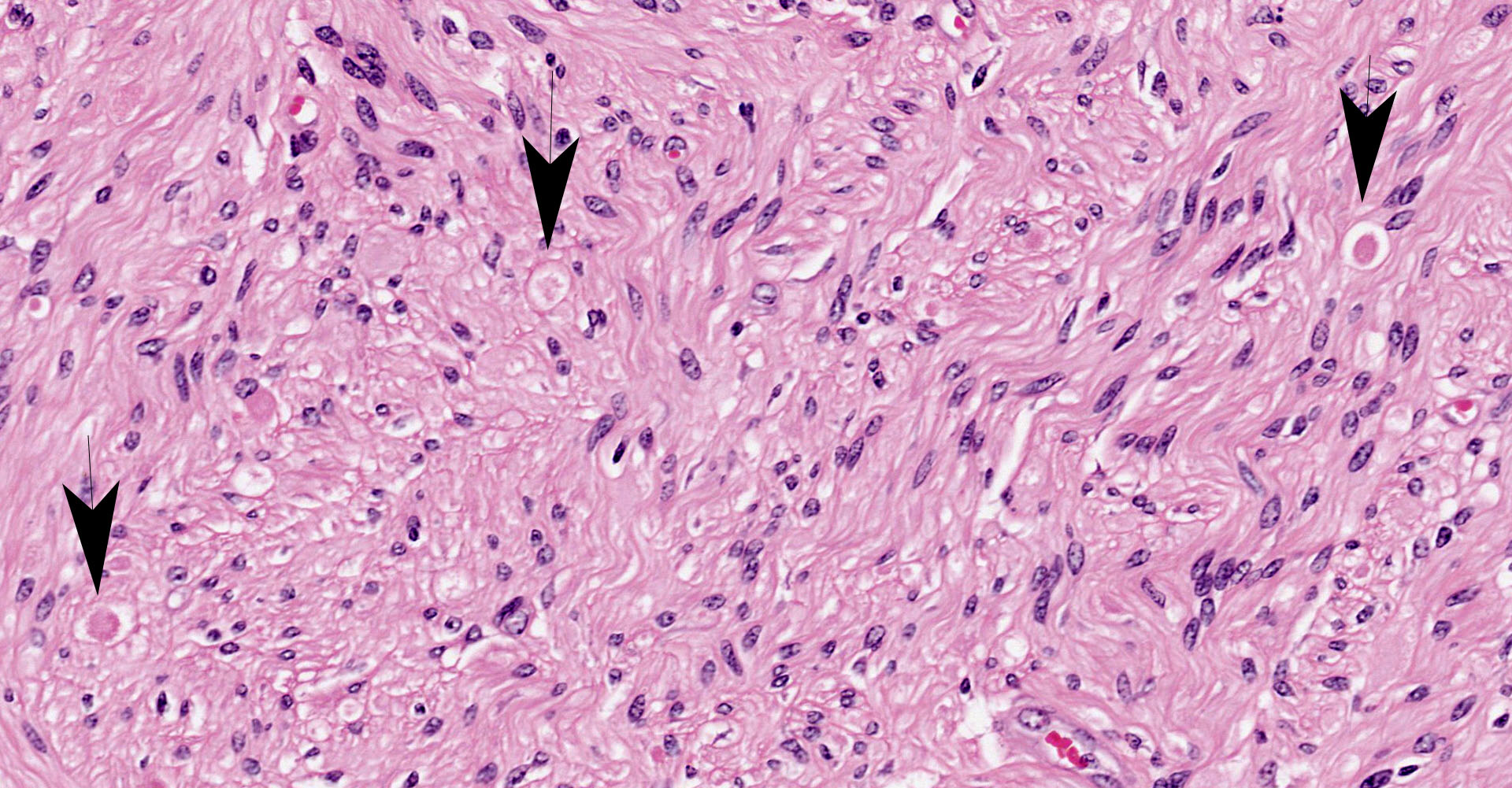
Coeliac/ cranial mesenteric ganglion. Multifocally, high numbers of neurons (estimated 70-80%) have degenerative changes characterized by central to complete cytoplasmic clearing of Nissl substance (chromatolysis), with an eccentrically positioned nucleus. Some of these neurons contain a central cytoplasmic eosinophilic body. Many neurons with chromatolytic changes have eccentric nuclei with condensed chromatin and shrunken nuclei (apoptosis). Axonal spheroids are seen occasionally between neurons. Multifocally, there is a mild increase in satellite cells. Rare individual and small clusters of lymphocytes are present scattered within the ganglion.
Contributor's morphologic diagnosis:
Coeliac/ cranial mesenteric ganglia: Neuronal chromatolysis, degeneration and apoptosis, multifocal, severe
Contributor's comment:
The presence of classical gross and histopathological features confirms the clinical suspicion of equine dysautonomia (equine grass sickness) in this case. Equine dysautonomia has a high mortality rate, and can have a significant impact in at risk areas.12 Acute, subacute, and chronic clinical presentations are recognized, and although these frequently overlap, this classification has utility in clinical decision making.6 Equine dysautonomia occurs most commonly in the United Kingdom,3 northern Europe, and South America (where it is referred to as "mal seco"),14 and has a strong association with grazing, with a peak incidence in the northern hemisphere during the spring and early summer months. Risk factors associated with the disease include recent movement to a new pasture, other cases of the disease on the same premises, pasture disturbance and a high number of horses. Younger animals are more often affected with a peak incidence in 2-7 year old horses.6 Equine dysautonomia is one example of a dysautonomia affecting domestic animals; others include the well characterized feline dysautonomia12 (Key Gaskell syndrome) and those occurring in dogs, sheep, rabbits and hares.1
All dysautonomias in domestic animals are typified by injury to the postganglionic sympathetic and parasympathetic neurons of the peripheral autonomic and enteric nervous system,8,12 resulting in failure of both sympathetic and parasympathetic innervation in several organ systems including the gastrointestinal tract.1 In the horse, functional obstruction of the gastrointestinal tract due to intestinal dysmotility is a significant and life threatening consequence.12 The result is gastric and small intestinal dilation, and impaction of the large intestine;14 both were evident in this case.
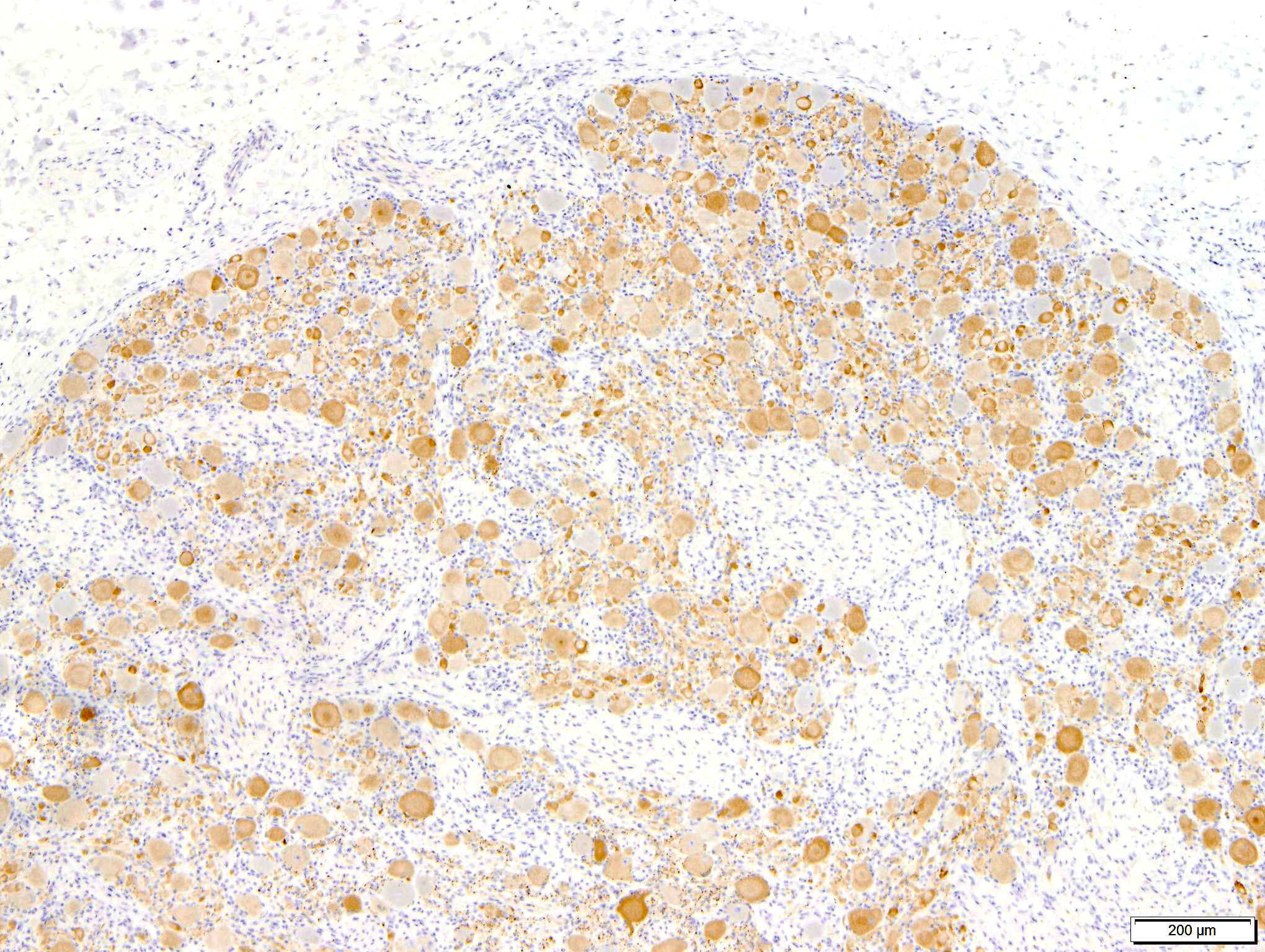
Characteristic neuropathological findings are extensive chromatolysis and neurodegeneration, with subsequent loss of neurons and degeneration and loss of dependent axons in autonomic ganglia of the peripheral nervous system.1 These changes were prominent in the section of coeliac/ cranial mesenteric ganglion submitted to the conference and also in the ganglia of the submucosal/ Meissner's and myenteric/ Auerbach's nerve plexuses of the ileum (not submitted to the conference), consistent with a previous report of the utility of this site in antemortem diagnosis of the disease.9 Additionally, immunohistochemical staining of the synaptic vesicle membrane protein synaptophysin showed marked cytoplasmic accumulation in neurons within both the coeliac/ cranial mesenteric ganglia and ileum. Intracytoplasmic synaptophysin accumulation, as demonstrated by immunohistochemistry, is recognized in dysautomonias of both horses and cats. Its use as a diagnostic marker has been demonstrated in horses and shows a staining pattern that is distinct from healthy control horses, colic cases and neuroparalytic botulism.2,16
A small increase in the numbers of non-neuronal satellite cells was observed in the section of coeliac/ cranial mesenteric ganglion examined. Reactive and proliferative changes in these elements occur with progression of the disease.1 Additionally the presence of multifocal small and loose aggregations of T lymphocytes and few individualized B lymphocytes in primarily perivascular locations (confirmed by immunohistochemistry for CD3 and PAX5 respectively) is also an unexpected and seemingly non-specific finding in this case as inflammation is not anticipated associated with neuronal degeneration and loss in equine dysautonomia.
The etiology of equine dysautonomia is currently unknown and is a focus of research into the disease, with Clostridium botulinum neurotoxins C and/ or D originating in the gastrointestinal tract (toxico-intestinal infection) hypothesized to be a possible cause.6,12 Low level serum antibodies to C. botulinum type C antigens is reported as a risk factor for the disease.6 This hypothesis has recently been tested in a C. botulinum type C vaccination trial in the United Kingdom,3,12 however the results are not available at the time of writing. An alternative etiology is the ingestion of a pasture derived mycotoxin,6,12 and a diverse range of other possibilities have also been investigated1 (for example heavy metal toxicity or plant derived toxins).
Accurate antemortem diagnosis is imperative to facilitate clinical decision-making; the most appropriate course of action is often prompt euthanasia, while limited number chronic cases do respond to medical management. Ileal biopsies, although highly sensitive and specific, require general anesthesia for abdominal surgery.6 In an attempt to circumvent this issue the utility of rectal biopsy has been assessed, however sensitivity and specificity have been suboptimal, possibly due the small number of cases examined and/ or to the lesser numbers of neurons present in the rectal submucosa.12 Most recently detection of ?-amyloid precursor protein (?-APP) in submucosal neurons of the rectal mucosa has been shown to be a sensitive and specific method of confirming equine dysautonomia,4 and examination of the neurons of the subgemmal plexus of gustatory papillae for changes suggestive of neuronal injury has also shown promise for use as a antemortem diagnostic test.7 Both offer the advantage of sample acquisition being relatively non-invasive.
Contributing Institution:
Division of Pathology, Public Health and Disease Investigation
Veterinary Diagnostic Services
School of Veterinary Medicine
College of Medical, Veterinary and Life Sciences
University of Glasgow (Garscube Campus)
464 Bearsden Road
Glasgow G61 1QH, Scotland
https://www.gla.ac.uk/schools/vet/cad/
JPC diagnosis:
Ganglion: Neuronal degeneration, multifocal, moderate, with chromatolysis, mild satellitosis, and lymphocytic ganglionitis.
JPC comment:
There was spirited discussion among participants regarding this case. While the neuronal vacuolation in this case is significant and a prominent feature, vacuolation of ganglial cells has been found in both healthy and affected animals.5 A short review of affected species that have been reported as being affected by dysautonomia include the horse, rabbit, cat, dog, hare, and human. While overo lethal white foal syndrome is a heritable trait and grass sickness appears to be acquired, the similar dysfunction of celiac/mesenteric ganglia results in similar clinical signs.
While there still exists a substantial knowledge gap regarding this disease, recent research may address certain specific aspects of predisposition, diagnosis, and treatment. A pedigree analysis of a population of Lipizzaner horses in Hungary showed a disproportionate number of horses affected by equine grass sickness were descendants of certain studs, with one having nearly 22% of offspring affected by the disease. While the true etiology of this disease is likely multifactorial, this suggests there may be a heritable component.15
Efforts to diagnose this disease antemortem have resulted in a preliminary scoring system, that incorporates epidemiologic data and clinical data. The most important factors identified in this framework include daily access to pasture and presenting from April to June, generalized or localized sweating, muscle tremors, and dysphagia. The score cutoff for this model results in a sensitivity of 100%, and a specificity of 53%. As additional risk factors are identified, the applicability of this model, as well as its specificity, may be improved.13
Cytology of scrapings from the cranial cervical ganglia of affected horses has resulted in a highly sensitive and specific method of diagnosis. Using a combination of May-Grunwald Giemsa (MGG), cresyl fast violet, ad hematoxylin and eosin, and using histologic sections of tissue as the good standard for diagnosis, independent pathologists agreed on diagnoses of equine dysautonomia. The primary limitation of cytology is the occasional specimen that contains too few neurons for evaluation.11
Though acute cases of equine dysautonomia are fatal, horses with chronic cases may survive with appropriate medical management. In chronic cases, the number of neurons found in the prevertebral and paravertebral ganglia, and in the enteric plexuses is lower than normal, but also lower than acute cases of dysautonomia. Additionally, small intestinal dilation, muscular hypertrophy, gastric mucosal hypertrophy and ulceration were common post-mortem findings in chronic cases. In most cases, the ileum was the most profoundly affected section of the gastrointestinal tract.10
References:
- Cantile C, Youssef S: Nervous System (Chapter 4). In: Maxie G, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals (Vol. 1). 6th ed. St. Louis, MO: Elsevier; 2016:333-334.
- Hilbe M, Guscetti F, Wunderlin S, Ehrensperger F. Synaptophysin: an immunohistochemical marker for animal dysautonomias. Journal of Comparative Pathology 2005;132(2-3):223-227.
- Ireland JL, McGorum BC, Proudman CJ, Newton JR. Designing a field trial of an equine grass sickness vaccine: A questionnaire-based feasibility study. Veterinary Journal 2016;213:64-71.
- Jago RC, Scholes S, Mair TS, Pearson GR, Pirie RS, Handel I, et al. Histological assessment of ? -amyloid precursor protein immunolabelled rectal biopsies aids diagnosis of equine grass sickness. Equine Veterinary Journal 2018;50(1):22-28.
- Jahns H, Fast C. A histopathological study of bovine ganglia. J Comp Path. 2014;150(2):234-244.
- McGorum BC, Pirie RS. Equine dysautonomia. Veterinary Clinics of North America - Equine Practice 2018;34(1):113-125.
- McGorum BC, Pirie RS, Shaw D, MacIntyre N, Cox A. Neuronal chromatolysis in the subgemmal plexus of gustatory papillae in horses with grass sickness. Equine Veterinary Journal 2016;48(6):773-778.
- Miller AD, Zachary JF: Chapter 14 Nervous System. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier; 2017:900 & 905.
- Milne EM, Pirie RS, McGorum BC, Shaw DJ. Evaluation of formalin-fixed ileum as the optimum method to diagnose equine dysautonomia (grass sickness) in simulated intestinal biopsies. Journal of Veterinary Diagnostic Investigation 2010;22(2):248-252.
- Milne EM, Pirie RS, Hahn CN, et al. A study of residual lesions in horses that recovered from clinical signs of chronic equine dysautonomia. Journal of Veterinary Internal Medicine. 2019;33(5):2302-2311.
- Piccinelli C, Jago R, Milne E. Ganglion Cytology: A novel rapid method for the diagnosis of equine dysautonomia. Vet Pathol. 2019;56(2):244-247.
- Pirie RS, McGorum BC. Equine grass sickness: an update. UK-Vet Equine 2018;2(1):6-10.
- Randleff-Rasmussen PK, Leblond A, Cappelle J, et al. Development of a clinical prediction score for detection of suspected cases of equine grass sickness (dysautonomia) in France. Veterinary Research Communications. 2018;42:19-27.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary System (Chapter 1). In: Maxie G, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals (Vol. 2). 6th ed. St. Louis, MO: Elsevier; 2016: 77.
- Vincze B, Varga M, Kutasi O, et al. Family aggregation analysis shows a possible heritable background of equine grass sickness (dysautonomia) in a Hungarian stud population. Acta Veterinaria Hungarica. 2020. https://doi.org/10.1556/004.2020.00038.
- Waggett BE, McGorum BC, Shaw DJ, Pirie RS, MacIntyre N, Wernery U, et al. Evaluation of synaptophysin as an immunohistochemical marker for equine grass sickness. Journal of Comparative Pathology 2010;142(4):284-290.