Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 25
1 May 2019
CASE IV: 12H5674 (JPC 4032439)
Signalment: A six year old, spayed female Pembroke Welsh Corgi dog (Canis lupus familiaris).
History: The dog presented with tachypnea, increased respiratory effort, cardiomegaly, panhypoproteinemia, mild anemia.
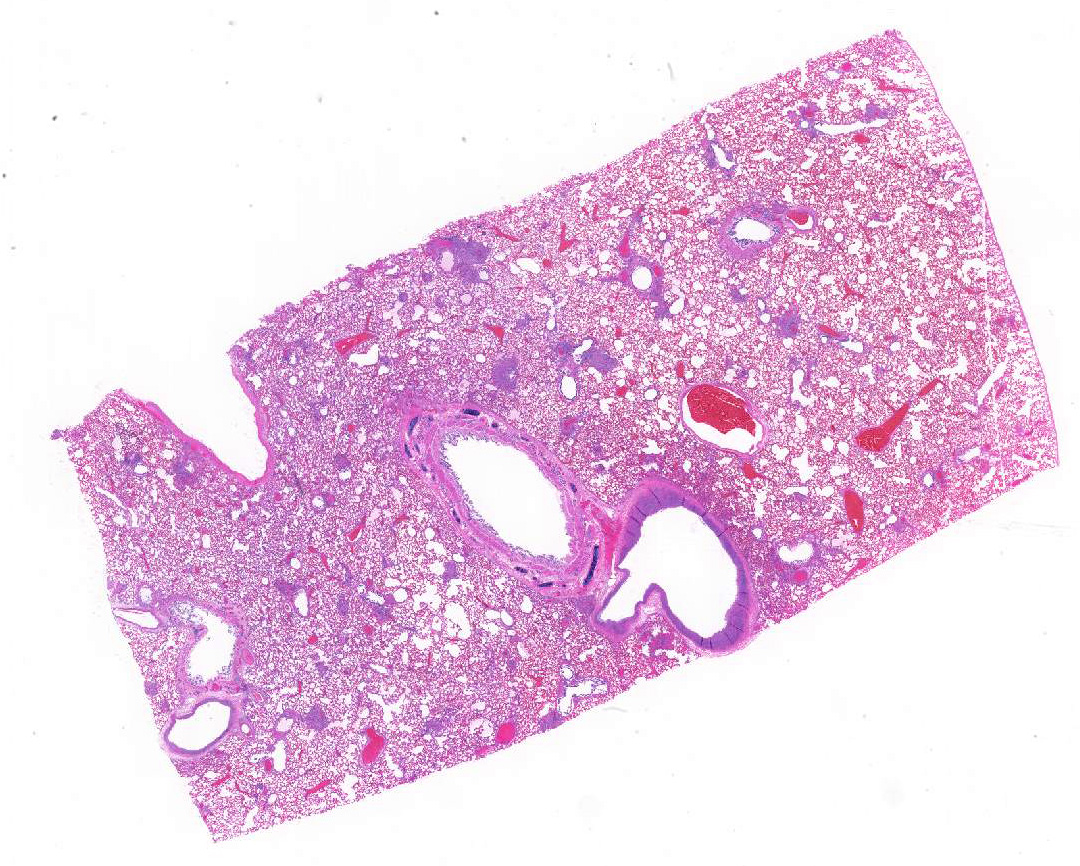
Gross Pathology: The dog had areas of ecchymoses around the peripheral veins and clotted blood in the right nasal cavity and ethmoid conchae. Feces were black and tarry. Lungs were diffusely firm and dark red. There were pinpoint multifocal white circular structures diffuse across the lung surface. Other findings included: subendocarial petechiae and petechiae cranial to the trigone of the bladder.
Laboratory results: The dog had a slight increase in neutrophils (11.81; range 3.0-11.4 x 10-3/ul), monocytes (2.35; range 0.15-1.35 x 10-3/ul), reduced hematocrit (36.9; range 37.0-55%), reduced platelets (43; range 200-500 x 10-3/ul), increased ALT (223; range 24-90 IU/L), BUN (>180; range 10-30 mg/dl), decreased calcium (8.6; range 9.7-11.3 mg/dl), increased phosphorus (10.7; range 3.2-6.0 mg/dl), increased glucose (202; range 68-115 mg/dl), decreased sodium (122; range 141-151 mEq/L), decreased total protein (4.6; range 5.2-7.1 gm/dl). v variable numbers of lymphocytes (plexiform pulmonary arteriopathy). Multifocal alveoli are variably-filled with seroproteinaceous fluid and/or hemorrhage along with increased numbers of alveolar macrophages. There is moderate multifocal to diffuse vascular congestion.
Contributor’s Morphologic Diagnoses:
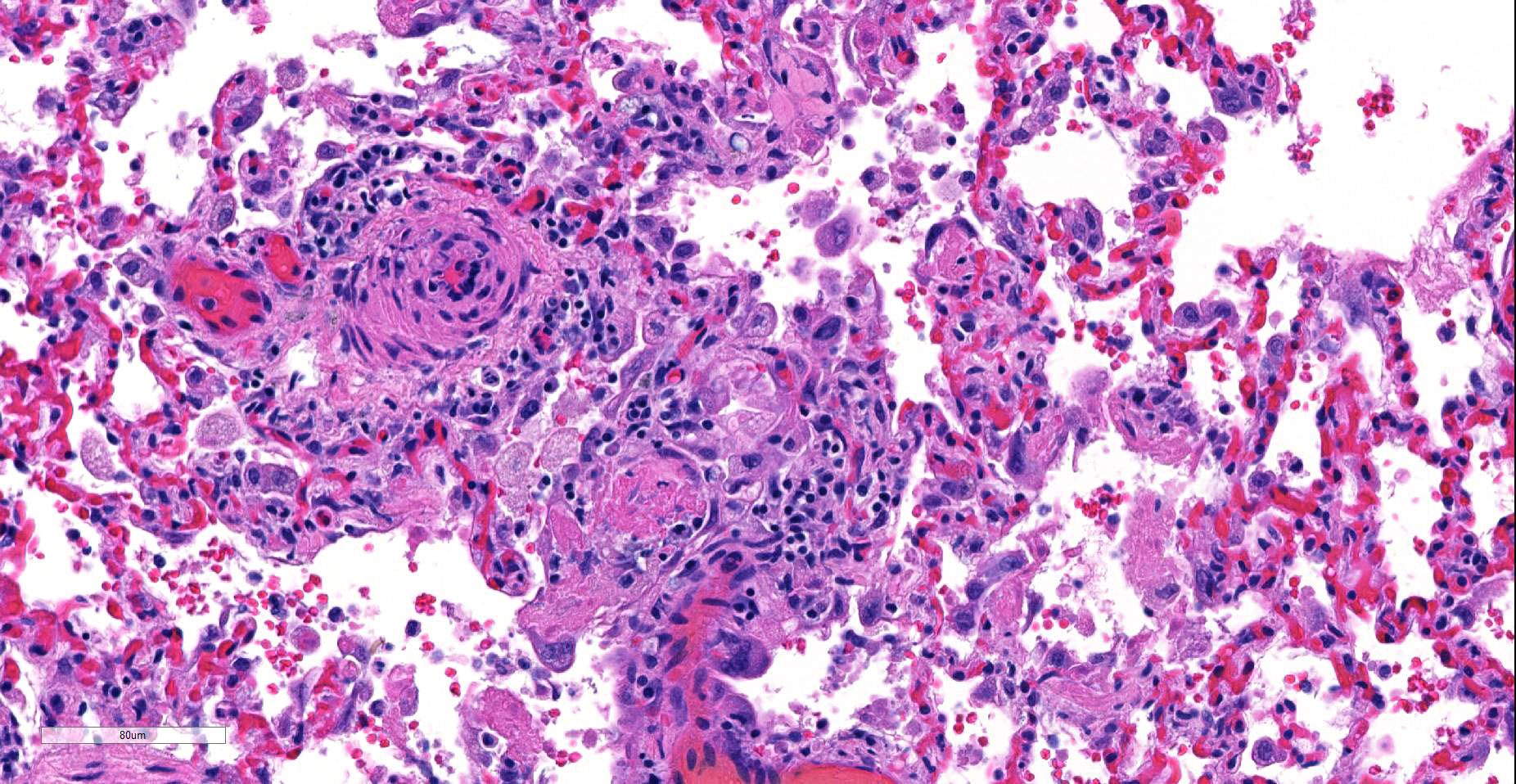
Lung, arteriolitis and arteritis, proliferative, lymphocytic and fibrosing with plexiform change, chronic moderate to severe Lung, hemorrhage, congestion, and edema, multifocal, moderate
Contributor’s Comment: The arterial lesions coupled with the clinical and laboratory results are suggestive of plexiform pulmonary arteriopathy.2,6 The dog lacked heartworms and lacked evidence of a left to right cardiac shunt and thus the changes are consistent with idiopathic pulmonary arterial hypertension (IPAH).6 In a limited number of dogs (six) with pulmonary arteriopathy and hypertension 67% were female, as in this case. The pulmonary vascular changes likely led to or were caused by pulmonary hypertension resulting in right heart failure suggested by radiographic and ultrasound findings that included right ventricular dilation and hepatic centrilobular congestion. Hemorrhage and congestion are present in the lung and are likely due to pulmonary hypertension possibly exacerbated by the thrombocytopenia. The hypoproteinemia and azotemia could not be fully explained. Right heart failure can lead to renal dysfunction but typically not glomerular leakage.
Some cases of IPAH have a mild inflammatory component in the arterioles which was evident in this case as well.6 It could not be determined if the inflammation was secondary or incidental to the proliferative change or if there is some antigen (self or infectious agent) that triggered the lymphocytic infiltration.
Contributing Institution:
Department of Veterinary Pathology
College of Veterinary Medicine
Iowa State University
Ames, Iowa 50010-1250
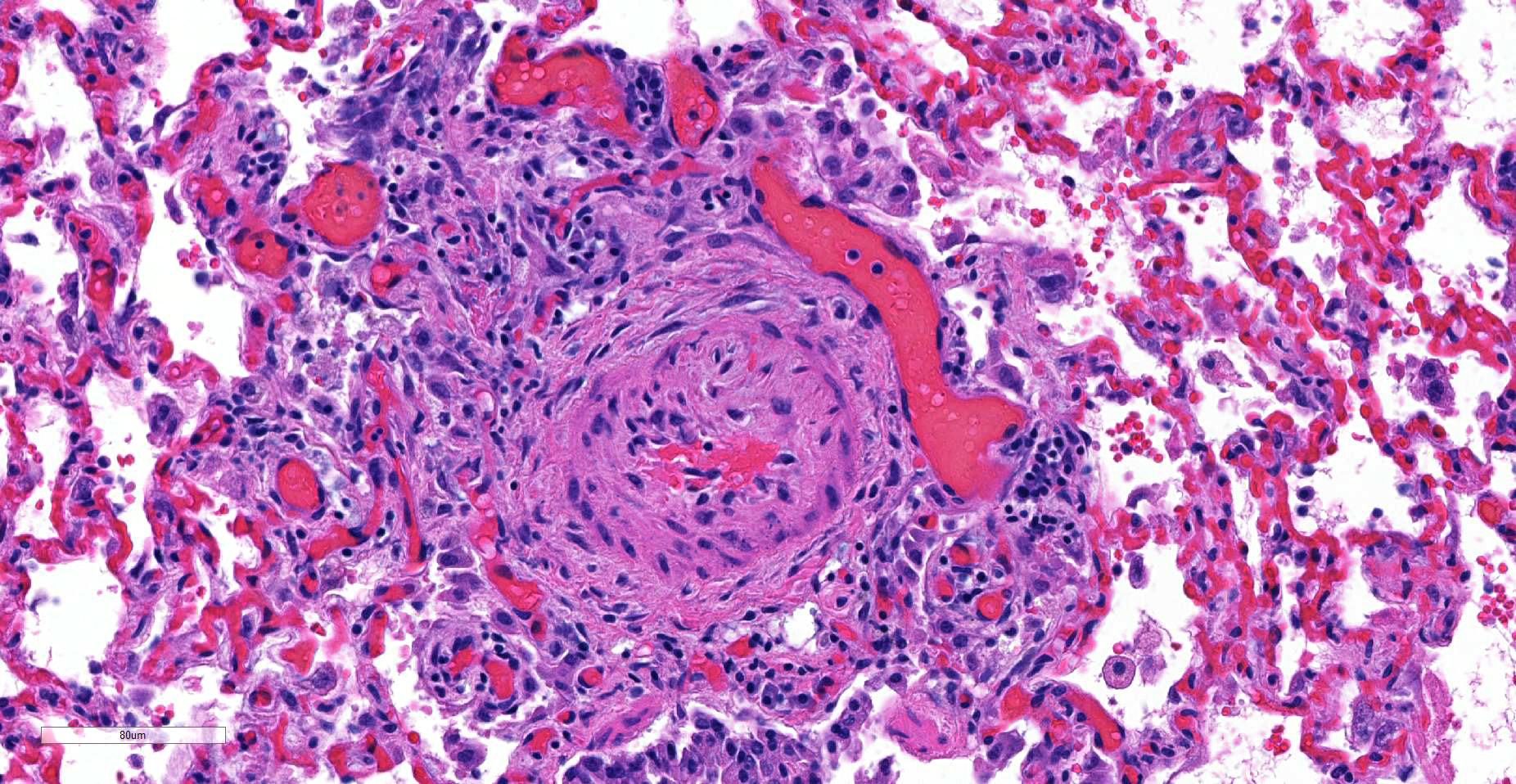
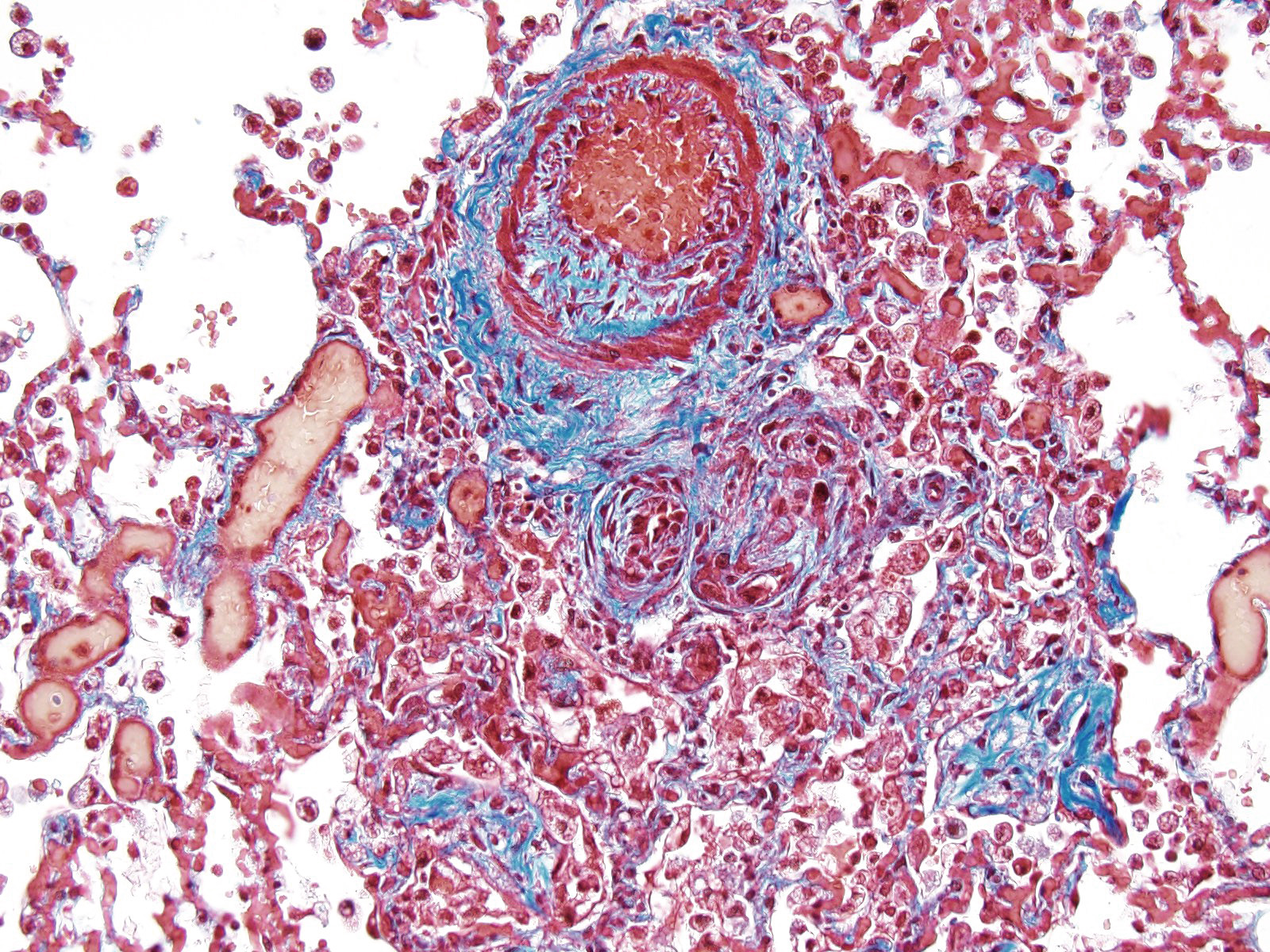
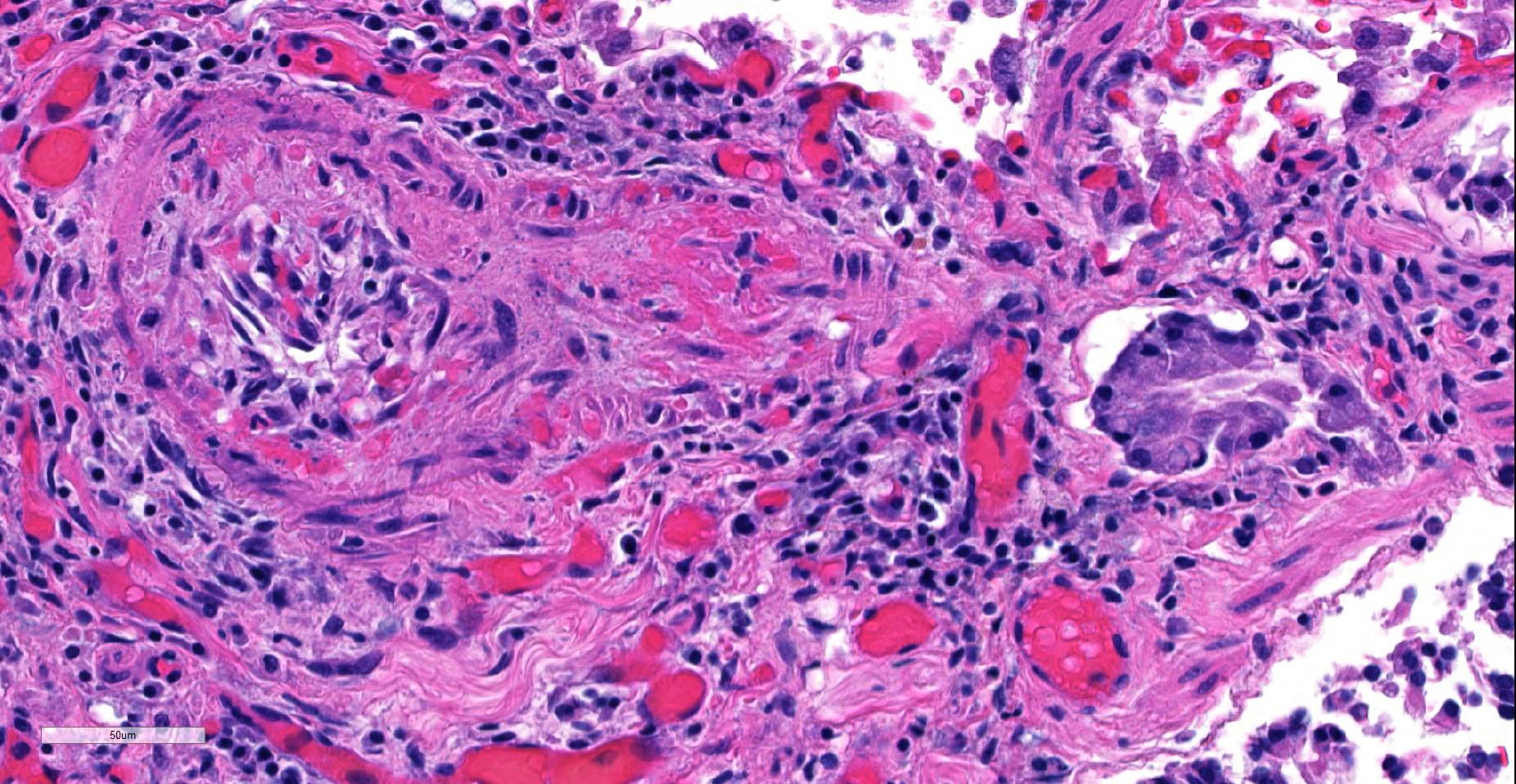
JPC Diagnosis: Lung, small arterioles: Plexiform (plexogenic) arteritis with marked intimal and medial hyperplasia and fibrosis, recanalization, and multifocal fibrinoid necrosis and exudative alveolitis.
JPC Comment: Plexiform (plexogenic) arterial lesions are striking histologic lesions which are associated with pulmonary hypertension in man and animals. In general terms, plexiform lesions is a form of “dilatation” lesion developing in late stages of pulmonary hypertension, following a precipitous decrease in pulmonary blood flow and resultant irreversible pulmonary hypertension. While many small arterioles will show evidence of prolonged hypertension, to include intimal hyperplasia, smooth muscle hyperplasia and disarray, and medial and adventitial fibrosis and in some cases fibrinoid necrosis (all present in this section), the plexiform lesion is a unique finding. This lesion is the result of a localized sac-like dilation of a branch of a muscular artery, in which fibrin accumulates from necrosis in the wall of the parent artery.1 Over time, this fibrin is organized into small vessels populated by cells from the parent artery as well. This pathogenesis also explains why the proliferating vessels are not circumferential but often present eccentrically on only one side of affected arterioles.1
These lesions have been well studied by sequential biopsies taken during the closure of congenital shunts in humans, and many of the associated lesions are seen in this section. The first lesion is increased muscularity of the wall of small arterioles (medial hypertrophy). This is often followed over time by an intimal cellular proliferation, in which smooth muscle cells penetrate the internal elastic lamina and their proliferation at this point may occlude the lumen. They are often accompanied by a proliferation of both collagen and elastin fibers within the intima as well.4 At this point, dilatation lesions will develop proximally and concurrent fibrinoid necrosis in the wall of the artery begins the final development of the plexiform lesion as described above.4
In humans, plexogenic arteriopathy may arise as a result of congenital cardiac shunting, but may also be seen with acquired cardiac shunts, hepatic cirrhosis with portal hypertension, schistosomiasis, and oral ingestion of drugs for anorexia.4
The first description of pulmonary plexiform arteriopathy (PPA), also in a Welsh Corgi, was published in 2004, and its etiology was attributed to an untreated patent ductus arteriosus (the animal was 21 months at its death.)2 PPA was also identified in 4 of 6 animals in another study of dogs with idiopathic pulmonary hypertension. Plexiform lesions were identified primarily at branching points of terminal to alveolar duct arteries.6
Plexiform lesions have also been identified in a line of rapidly growing broiler chickens which had a high incidence of idiopathic pulmonary arterial hypertension; however, as opposed to other species, plexiform lesions could be identified immediately post-hatch and did not require a period of pulmonary arterial hypertension.5 A line of genetically engineered mutant mice that develop plexiform arteriopathy has been developed as a result of decreased expression of intersectin-1s.3
References:
- Heath D, Smith P. Plexiform lesions with giant cells. Thorax 1982; 37:394-395.
- Kolm US, Amberger CN, Boujon CE, Lombard CW. Plexogenic pulmonary arteriopathy in a Pembroke Welsh Corgi. J Small Anim Prac 45(9):461-466, 2004
- Patel M, Predescu D, Bardita C, Chen J, Jegnathan N, Pritchard M, DiBartolo S, Machado, R, Predescu S. Modulation of intersectin-1s lung expression induces obliterative remodeling and severe plexiform arteriopathy in the murine pulmonary vascular bed. Am J Pathol 2017; 187(3):529-542
- Wagenvoort CA. Plexiform arteriopathy. Thorax 1994: 49:S39-245.
- Wideman RF, Mason JG, Anthony NB, Cross D. Plexogenic arteriopathy in broiler lungs: Evaluation of line, age and sex influences. Poultry Sci 2015; 94:628-638.
- Zabka TS, Campbell FE, Wilson DE. Pulmonary arteriopathy and idiopathic pulmonary arterial hypertension in six dogs. Vet Pathol 43(4): 510-522, 2006