Signalment:
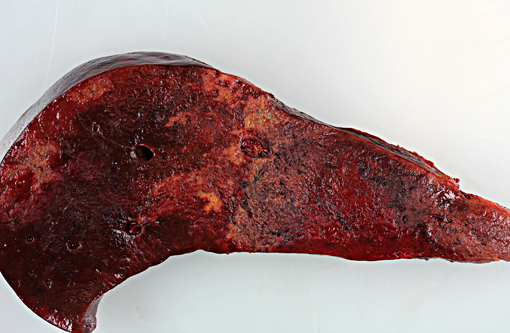
Gross Description:
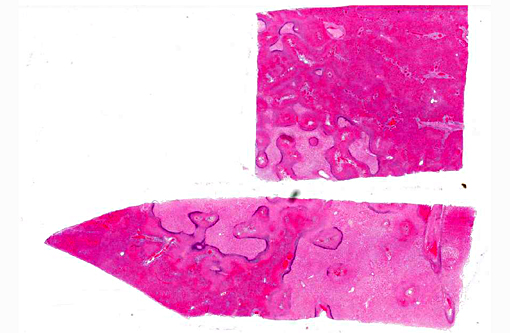
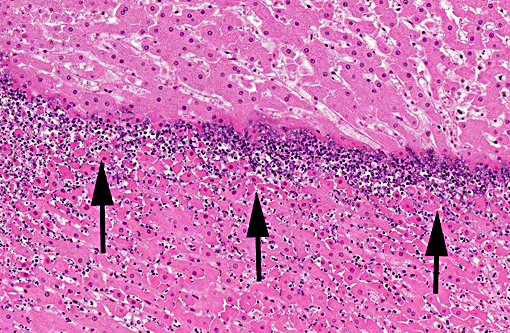
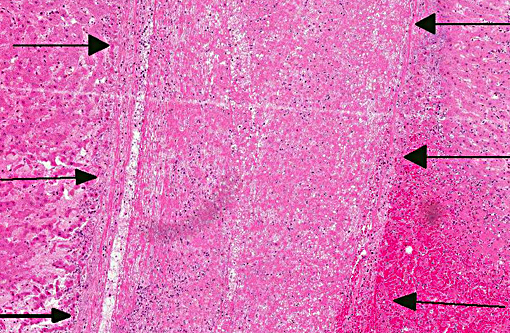
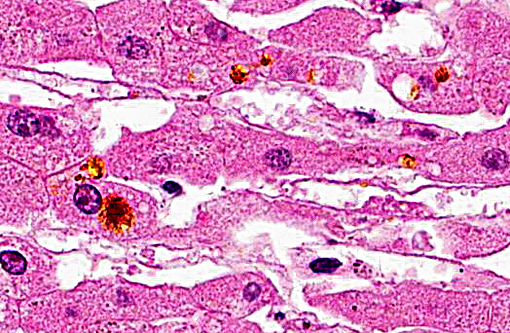
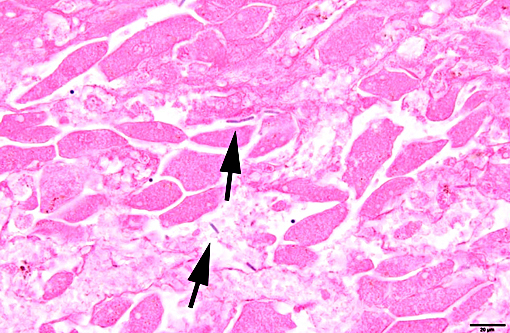
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
There is considerable overlap in the gross pathology, histopathology and pathogenesis of these diseases. Black disease is caused by C. novyi type B, a bacterium that produces potent alpha and beta toxins. Bacillary hemoglobinuria is caused by C. haemolyticum (formerly C. novyi type D) which produces beta toxin only. Both diseases are characterized by acute hepatic necrosis and other systemic lesions associated with toxemia and generalized vascular damage.(5) As the name suggests, bacillary hemoglobinuria is further characterized by intravascular hemolysis with anemia and hemoglobinuria.(6) The pathogenesis of both diseases begins with the ingestion of environmental spores with seeding to histiocytes within the liver, spleen, and bone marrow. Spores lie dormant in the liver until the formation of a localized anaerobic environment allowing for germination of spores and the production of potent exotoxins by vegetative bacteria. In ruminants, migration of the common liver fluke, Fasciola hepatica, is thought to be the initiating event.(6)
To date, there are 7 reports of clostridial hepatitis in equids occurring in Australia, New Zealand, the United Kingdom, and the United States.(3,4,6,7,9) Similar to the current case, clinical disease in horses is characterized by acute onset of depression, fever, abdominal pain, icterus, tachycardia, and tachypnea with rapid deterioration and death in 12-48 hours.(7,9) Successful therapy has not been described and is not surprising given the rapid course of disease and difficulty in establishing an antemortem diagnosis. Reported necropsy findings include serosanguinous pericardial, pleural and peritoneal effusions, serosal hemorrhages, icterus, fibrinous peritonitis and hepatic necrosis.(3,4,7,9) The inciting cause of the suitable anaerobic conditions for spore germination within the equine liver has not been definitively determined. Strongyle migration though the liver is an inconsistent finding and in the current case, there was no clear evidence of larval migration.(5,9) Interestingly, many of the reported cases have a recent history of anthelminthic therapy prior to the onset of clinical signs.(3,5,9) Recent use of anthelmintics was not reported in the current case.
Arriving at an etiologic diagnosis was problematic in the current case. Liver was submitted to two laboratories for anaerobic culture and fluorescent antibody testing (FAT) for Clostridium spp. Clostridium novyi group organisms were not detected by FAT or by culture, highlighting the challenge at arriving at an etiologic diagnosis in cases of clostridial hepatitis. Both C. novyi and C. haemolyticum are extremely oxygen sensitive and fastidious in their nutritional requirements making culture challenging and an unreliable diagnostic tool.(5,9) This diagnostic challenge is further highlighted by a report wherein C. novyi type A isolates were sent to 669 laboratories worldwide as part of an external quality control program. Only 3.5% of laboratories made a definitive identification of C. novyi type A.(2) In the current case, immunohistochemistry was positive for C. novyi and was instrumental in confirming the diagnosis.
JPC Diagnosis:
Conference Comment:
The differential diagnosis includes equine serum hepatitis, which is a common cause of acute liver failure in young horses. Grossly in this condition the liver is described as flaccid (dishrag liver) with a mottled or reticular pattern on cut section. Histology is characterized by extensive hepatic necrosis with stromal collapse; remaining hepatocytes often demonstrate fatty degeneration. This is typically not an acute process and mild fibroplasia is often present, which is in contrast to the liver seen in this case, although the clinical course has some similarities.(1) Not knowing the age of this animal, another infectious rule out is C. piliforme, the cause of Tyzzers disease, which is reported in young foals. In contrast to the lesion in this case, the microscopic lesion of Tyzzers consists of random foci of coagulative necrosis with neutrophilic infiltrates. The diagnosis depends on seeing the organisms at the periphery of necrotic areas within degenerate and normal appearing hepatocytes, and the organisms often appear in bundles.(1) Salmonella typhimurium was also mentioned as a possibility, but again is most often seen in foals. Lesions are most commonly associated with the intestine, particularly in adult horses, but in cases of septicemia lesions may be seen in the liver.(8)
Since infectious organisms were not apparent in most slides, many conference participants considered a toxic etiology such as blue-green algae. Microcystin-LR is the most commonly referenced cyanobacterial hepatotoxin, found on ponds or other small bodies of water after a seasonal bloom. It is taken up into hepatocytes where it inhibits cytoplasmic protein phosphatases leading to necrosis, apoptosis, and perisinusoidal hemorrhage. The pattern of necrosis is most often centrilobular to massive, but can vary depending on the individual animal and amount of toxin ingested.(1) Overall, this was a challenging case with clinical, gross and histologic characteristics consistent with several potential etiologic agents.
References:
1. Cullen JM, Stalker MJ. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 3. 6th ed. St. Louis, MO: Elsevier; 2016:312-331.
2. Finn SP, Leen E, English L, OBriain DS. Autopsy findings in an outbreak of severe systemic illness in heroin users following injection site inflammation. Arch Pathol Lab Med. 2003; 127:1465-1470.
3. Gay CC, Lording PM, McNeil P, Richards WPC. Infectious necrotic hepatitis (black disease) in a horse. Equine Vet J. 1980;12(1):26-27.
4. Hollingsworth TC, Green VJD. Focal necrotizing hepatitis caused by Clostridium novyi in a horse. Aus Vet J. 1978;54:48.
5. Oaks JL, Kanaly ST, Fisher TJ, Besser TE. Apparent Clostridium haemolyticum/Clostridium novyi infection and exotoxemia in two horses. J Vet Diagn Invest. 1997;9:324-325.
6. Stalker MJ, Hayes MA. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 2. 5th ed. London, UK: Saunders Elsevier; 2007: 354-356.
7. Sweeny HJ. Infectious necrotic hepatitis in a horse. Equine Vet J. 1986;18(2):150-151.
8. Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie GM, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 3. 6th ed. St. Louis, MO: Elsevier; 2016:172-174.
9. Whitfield LK, Cypher E, Gordon SJG, et al. Necrotic hepatitis associated with Clostridium novyi infection (black disease) in a horse in New Zealand. N Z Vet J. 2015;63(3):177-179.