Signalment:
Gross Description:
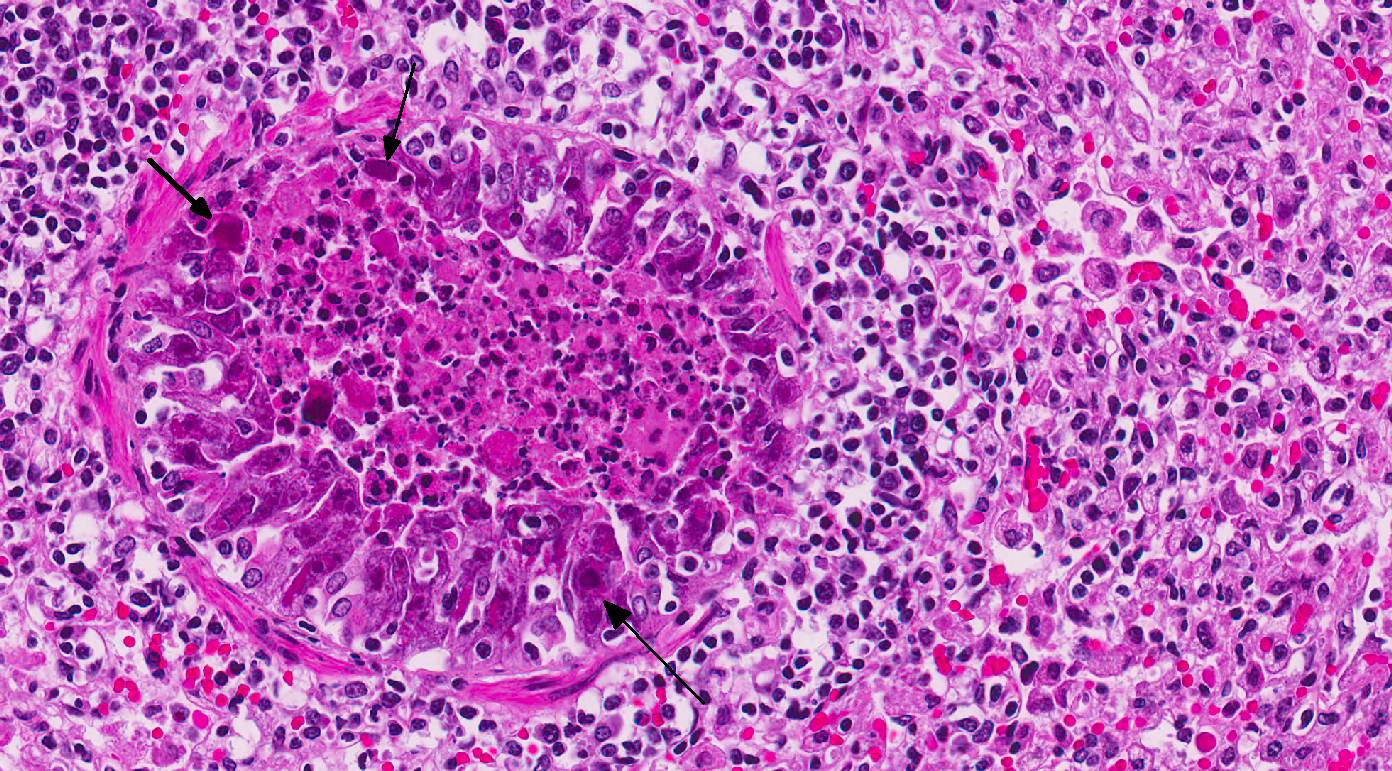
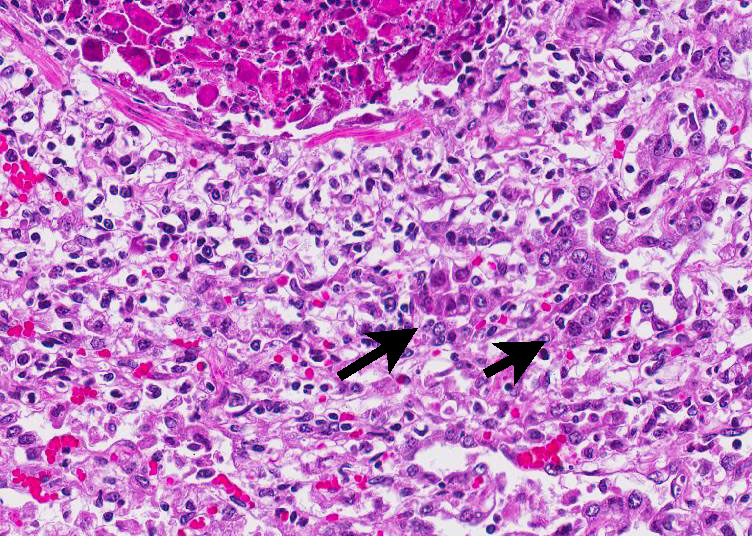
Histopathologic Description:
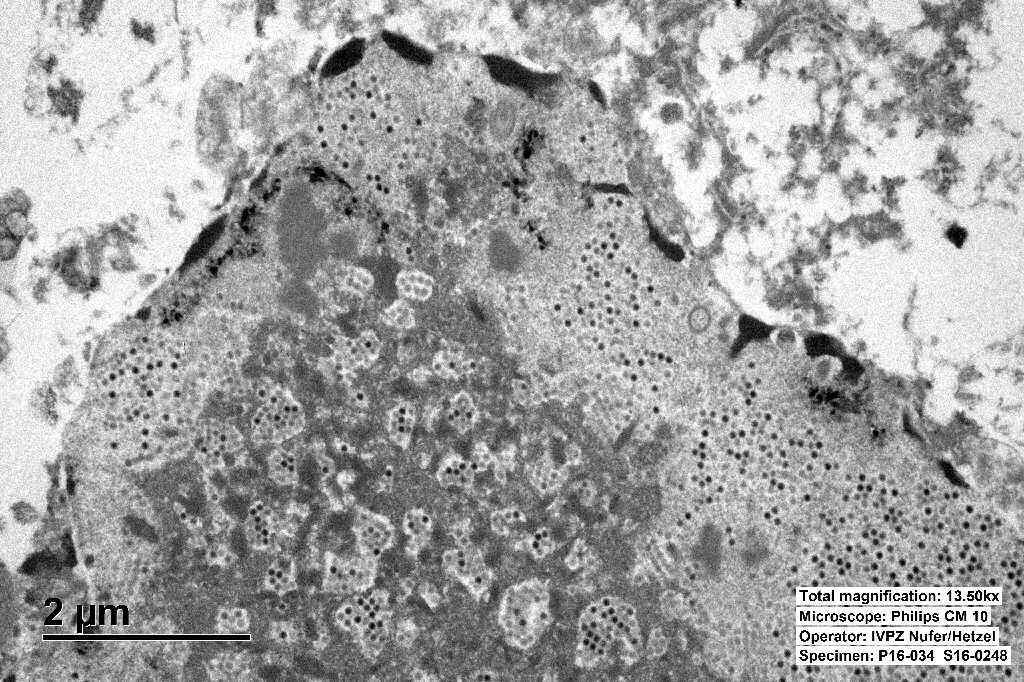
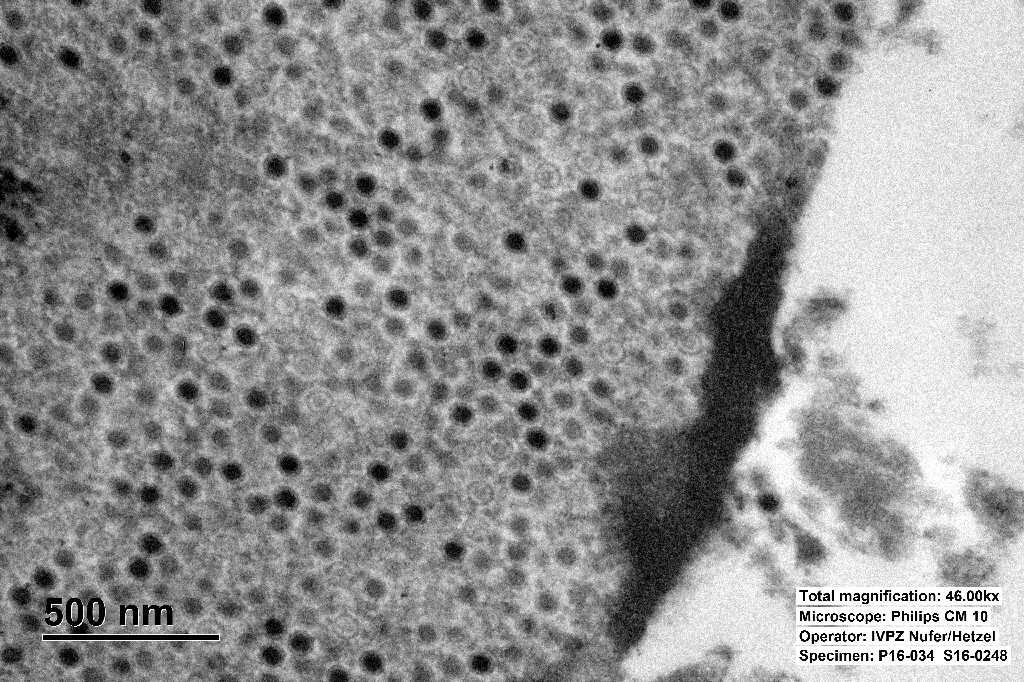
The inclusions are typical for adenoviruses and the diagnosis was confirmed by subsequent transmission electron microscopy.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Adenovirus virions are non-enveloped, hexagonal, and have an icosahedral symmetry, 70-90 nm in diameter, with one (genus Mastadenovirus) or two (genus Aviadenovirus) fiber projections from each vertex of the capsid.2,3
The genome consists of a single linear molecule of double-stranded DNA, 26 to 45 kbp in size with inverted terminal repeats. Infection may be productive, abortive, or latent. In productive infections, the viral genome is transcribed in the nucleus, mRNA is translated in the cytoplasm, and virions self-assemble in the nucleus. In latent infections and in transformed and tumor cells, viral DNA is integrated into the host genome.2,3 Intranuclear inclusion bodies are formed, which contain numerous virions in paracrystalline arrays.2,4
Adenoviruses have a narrow host range and many adenoviruses cause persistent infections. Reactivation of a latent infection can take place by immunosuppression, e.g. Equine adenovirus causes severe disease in immunocompromised hosts.3
In dogs two types of canine adenoviruses are well known, type 1 and 2. Type 1 (CAdV-1) causes infectious canine hepatitis, a potentially fatal disease involving vasculitis and hepatitis. Type 1 infection can also cause respiratory and eye infections. Canine adenovirus 2 (CAdV-2) is one of the potential causes of kennel cough. Core vaccines for dogs include attenuated live CAdV-2, which produces immunity to CAdV-1 and CAdV-2. CAdV-1 was initially used in a vaccine for dogs, but corneal edema was a common complication.3
In horses, adenoviral infections are usually asymptomatic or cause a mild upper respiratory disease. In Arabian foals with a primary severe combined immunodeficiency disease, equine adenovirus 1 can cause bronchopneumonia and generalized disease, involving pneumonia and destruction of pancreatic and salivary gland tissue.3
In ruminants, bovine adenovirus-3 causes mild respiratory disease. Occasionally calves suffer from a severe respiratory or enteric disease.3
In sheep, adenoviral infections occasionally cause cytomegalic bronchiolar and alveolar epithelial cells similar to cytomegalovirus. Lambs can suffer from a severe respiratory or enteric disease.3
In non-human primates (NHPs) it can cause mild to moderately severe respiratory and enteric disease and keratitis/conjunctivitis.3
The avian adenoviral infections are divided into groups. Group I causes inclusion body hepatitis, respiratory and enteric infections in chickens, turkeys, and geese. Group II causes turkey hemorrhagic enteritis, marble spleen disease. And group III causes egg drop syndrome in chickens and ducks.3
In humans, an adenoviral infection causes a respiratory disease, pharyngitis, and follicular conjunctivitis.3
Some adenoviruses have shown to be oncogenic in laboratory animals. Cells from a number of rodent species and humans can be transformed in culture by adenoviruses. The frequency of malignant transformation is extremely low. Some adenoviruses, such as Ad2 and Ad5, have not been shown to be oncogenic in animals. Tumorigenic potential has been attributed to the capacity of some adenoviruses to turn off the expression of genes of the major histocompatibility complex and thus to allow the transformed cells to overcome host defenses and grow into solid tumors. 2,5
Viral pneumonia caused by adenovirus is one of the most common infections in breeding colonies and collections of guinea pigs. Guinea pigs are susceptible to a respiratory adenovirus that causes pulmonary disease and inclusions in respiratory epithelium. Guinea pig adenovirus (GPAdV-1) has been reported in Europe, North America, and Australia. The infection is characterized by a low morbidity, but drastically high (up to 100%) mortality rate.1 Incubation time varies between 5 to 10 days. The animals die within 24 to 48 hours after showing the first clinical signs.3 Clinical disease most often appears in young animals. Affected animals may show no clinical signs or can be severely dyspneic. The characteristic macro-scopic lesions involve a consolidation of the cranial lobes of the lungs and hilus.1,4
A diagnosis may be confirmed by immuno-cytochemistry, serology, PCR, or the presence of adenoviral particles in affected cells by electron microscopy. Recently, a homotypic antigen has been developed using the GPAdV hexon gene incorporated into replication-defective adenovirus vector. This assay has proven to be more sensitive than the mouse adenovirus (MAV), which was used in the past for serological testing of GPAdV.1
JPC Diagnosis:
Conference Comment:
In this case, conference participants readily identified the prominent and highly characteristic intranuclear amphophilic inclusions within the exfoliating bronchial and bronchiolar epithelial cells, along with moderate to severe peribronchial leukocyte infiltration. Typically, adenoviruses are associated with prior immune suppression.2,3 In Guinea pig adenoviral infection (GPAdV-1), the mortality rate in clinically affected animals is nearly 100%, with young and immunosuppressed animals being the most affected. Typical lesions have been observed in the airways of clinically normal animals, emphasizing the importance of immune suppression in this disease entity. Some participants posited that there may be a secondary bacterial infection that contributed to the pulmonary changes in this Guinea pig.5 Viruses are known to damage the respiratory epithelium, which plays a role in the pathogenesis of bacterial pneumonia by inhibiting bacterial clearance and facilitating adhesion.2,3,5 In this case, there is clear epithelial targeting of the bronchial and bronchiolar epithelium with suppurative exudate filling airways and extending into the surrounding interstitium. However, no additional agents were detected by the contributor. Conference participants discussed the differentials for severe respiratory disease in Guinea pigs, including: parainfluenza virus, Guinea pig cytomegalovirus, and bacterial pneumonia of the lower respiratory tract, such as Bordetella bronchiseptica.5 Definitive diagnosis is made by immunohisto-chemistry, PCR, or the demonstration of the adenovirus particles in affected cells by transmission electron microscopy (TEM).5
Ultrastructurally, adenoviral inclusions are composed of prominent intranuclear paracrystalline arrays of virions and unassembled viral capsid proteins, demonstrated nicely by the TEM image provided by the contributor. Adenovirus is not the only virus that will form hexagonal paracrystalline arrays in affected tissue. Conference participants discussed other viruses that form ultrastructural para-crystalline arrays. These include poly-omavirus, papillomavirus, picornavirus, iridovirus, and circovirus.3
References: