WSC 22-23
Conference 11
Case I:
Signalment:
Adult female giant Pacific octopus (Enteroctopus dofleini)
History:
Octopus arrived to the National Aquarium in Baltimore bright, alert, and responsive, but missing multiple distal limb pieces, suspected to be traumatic in nature. Two weeks after capture, this octopus was noted to be hyporexic. Fecal examination was concerning for cystic structures, possibly Aggregata sp. Octopus was noted to be dark in color and posturing strangely the next morning, reportedly standing on its limbs. The animal was found deceased after lunch; lack of heartbeat was confirmed via Doppler and ultrasound. Prior to death, water temperature was noted to be within normal limits and dissolved oxygen content at 99%.
Gross Pathology:
The submitting institution performed the gross examination which identified partial traumatic amputation of the distal arms of R1-3, L2, and L4, as well as marked gill pallor. Hemolymph drawn after death was clear when drawn, but turned deep blue (aerated) after 1 minute in the collection tube. The kidneys were also said to contain multiple pinpoint white spots of unknown significance.
Laboratory Results:
Below are values from the aforementioned post-mortem sample; published reference intervals (RI) for hemolymph in octopus are not available.
Hemocyte count: 2.255k/uL (empirically low)
Copper: 174.925 mcg/dL (likely appropriate, RI unknown)
Chemistry: Glu 20 mg/dL (empirically low), Na 376.2 mmol/L (likely appropriate, RI unknown), K 12.6 mmol/L (empirically high), Cl 402 mmol/L (empirically high), TP 11.4 g/dL (empirically very high)
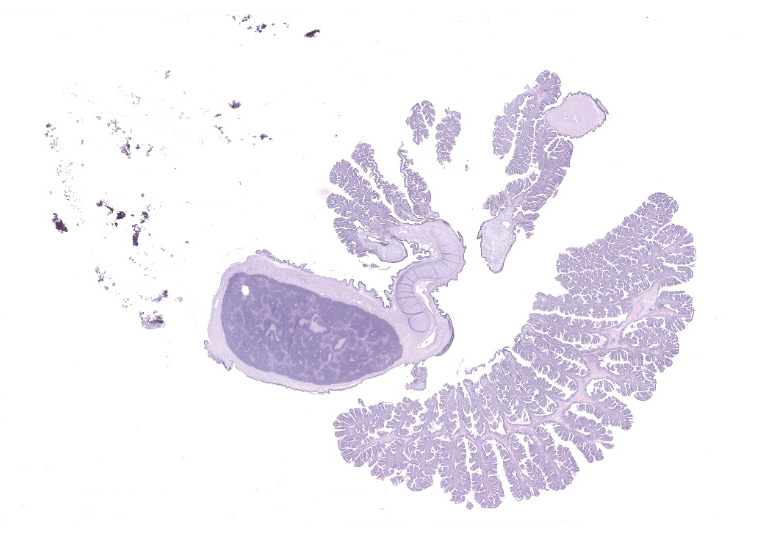
Microscopic Description:
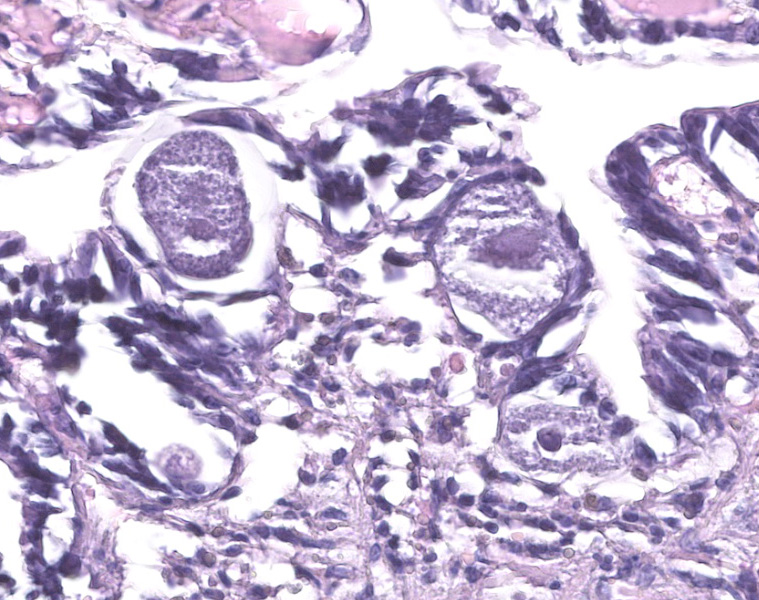
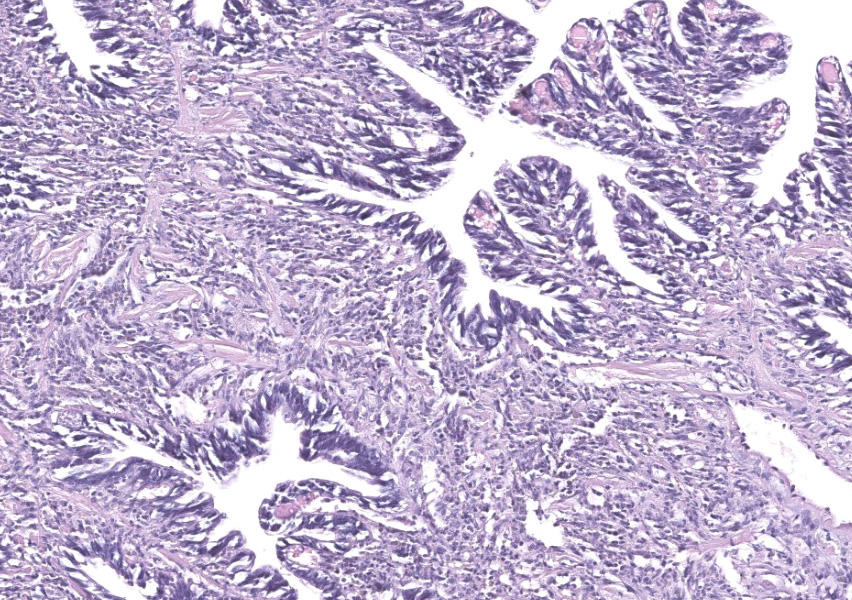
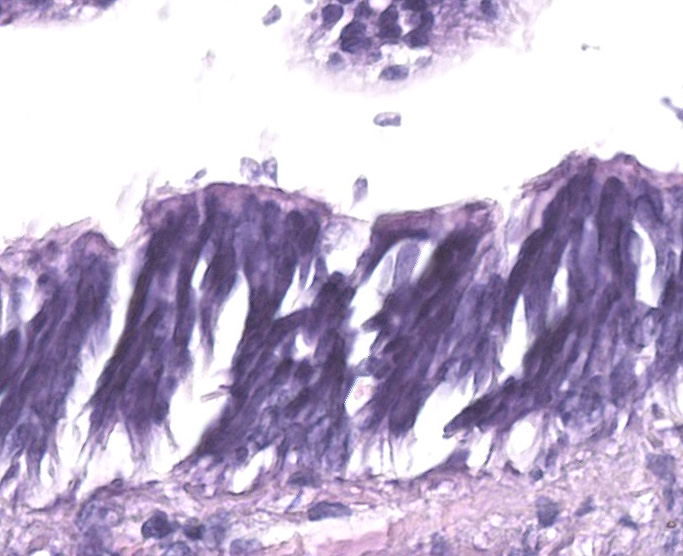
Gill: Moderate numbers of macrogametes are embedded in the connective tissue of the gill lamellae. These round to ovoid cystic structures, with a central nucleus and a large, darkly staining nucleolus, measure up to 100 ?m in diameter and frequently invade the gill epithelium. Cysts contain abundant finely granular or vacuolated basophilic cytoplasm. Hemocytic infiltration of the affected gill lamellae is variable, with some areas of severe hemocytic inflammation noted in the interstitium of the gill lamellae. Hypertrophy of infected epithelial cells is quite prominent in some regions. The centralized branchial gland is unaffected. In some sections, low numbers of flagellate protozoa are noted adherent to the apical surface of gill epithelial cells, consistent with Ichthyobodo sp.
Contributor?s Morphologic Diagnoses:
Gill lamina: coccidiosis, multifocal, moderate with intralesional macrogametes.
Contributor?s Comment:
The eimeriorin coccidian protozoal organisms of the genus Aggregata and the greater phylum Apicomplexa are frequently found to infect the digestive tract of a diverse number of cephalopods. These parasites can cause damage to the host tissue including direct mechanical injury, as well as indirect enzymatic and innate immune effects.2 This leaves cephalopods under considerable stress, most commonly those housed in fishery or aquaculture situations, vulnerable to secondary infections. Additionally, it has been reported that the enzymatic disturbances that occur in octopods result in malabsorption, producing a gradual decline in protein levels, emaciation, and even death.3 In the past 25 years, Aggregata spp. organisms have been considered the most important disease-producing infectious agent in both wild and cultured populations of the common octopus (Octopus vulgaris), a major protein source in many fish-eating countries.4 Infections have previously been described in the intestinal tracts of giant Pacific octopuses (Enteroctopus dofleini) from this colony as the result of a newly identified species, Aggregata dobelli.8
Aggregata spp. coccidia have heteroxenous development with the asexual stages (merozoites, meronts) invading the intestinal epithelial cells of crustaceans, the intermediate host, until subsequent ingestion by cephalopods.2 These merogonial stages can then infect the digestive tract of the definitive host, as well as the epithelial cells of other tissues, such as the gill lamellae. To gain access to these extraintestinal sites of infection, the invasive oocysts are able to migrate through tissues, causing hypertrophy of invaded cells, hemocytic infiltration, and, consequently, phagocytosis by these hemocytes.5 Oocyst and macrogamete stages are frequently observed embedded in the connective tissue and surrounded by a thin cyst wall or a multilayered dark membrane. In some cases, abundant developmental stages may be accompanied by desquamation and necrosis of the gill epithelium.
Detection of coccidiosis may be particularly important in octopods caught from the wild for rearing in aquaculture or exhibition in aquariums. Fecal oocyst count is the most common method of detection, although there are no standardized ranges of normal recognized in cephalopods. Determination of the species relies on the morphological characterization of the size, shape, and number of sporozoites in each sporocyst, as well as the ornamentation.2 For example, Aggregata dobelli has a smooth-surfaced sporocyst containing 9-22 sporozoites. The positive identification of this developmental stages requires histological examination of the digestive tract, which cannot be performed in living animals. Treatment also presents a significant dilemma. While protozoan infections in aquarium species are typically controlled through the addition of ionic or chelated copper, high concentrations of formalin, or chloroquine diphosphate solutions to closed tanks, these methods are unsuitable for use on octopods.1 Thus, the ongoing study of coccidiosis in cephalopods requires new methods of both molecular detection and eradication of these parasites.
Contributing Institution:
Johns Hopkins School of Medicine Department of Molecular & Comparative Pathobiology (https://mcp.bs.jhmi.edu/)
JPC Diagnosis:
Gill: Branchitis, hemocytic, diffuse, moderate, with numerous intraepithelial macrogametocytes (etiology consistent with Aggregata sp.) and few surface flagellates (etiology consistent with Ichthyobodo sp.).
JPC Comment:
The contributor provides an excellent overview of Aggregata, a very important pathogen of octopuses. Another organism featured in this case is Ichthyobodo, a free swimming flagellate which can infect freshwater and marine fish and octopuses.7 The flagellate, which measures 6-10 ?m, attaches to and penetrates epithelial surfaces, especially the gills.7 Gross lesions are typically minimal, and histologic lesions range from absent to severe bronchitis with epithelial degeneration and necrosis.6,9 The parasites may not be seen histologically as they quickly depart the host after it dies.9 In a recent survey of 19 wild-caught giant Pacific octopuses which died captivity, ten of the animals had Ichthyobodo infections, nine of which had severe bronchitis.6 In this case, Ichthyobodo is present in low levels and was probably not pathogenic to the patient.
References:
- AZA Aquatic Invertebrate Taxon Advisory Group (AITAG). Giant Pacific Octopus (Enteroctopus dofleini) Care Manual. Association of Zoos and Aquariums; 2014.
- Castellanos-Martínez S, Gestal C, Pascual S, Mladineo I, Azevedo C. Protist (Coccidia) and Related Diseases. In: Gestal C, Pascual S, Guerra Á, Fiorito G, Vieites J, eds. Handbook of Pathogens and Diseases in Cephalopods. 1st Springer, Cham; 2019.
- Gestal C, Páez de la Cadena M, Pascual S. Malabsorption syndrome observed in the common octopus Octopus vulgaris infected with Aggregata octopiana (Protista: Apicomplexa). Diseases of Aquatic Organisms, 2002; 51: 61-65.
- Gestal C, Guerra A, Pascual S. Aggregata octopiana (Protista: Apicomplexa): a dangerous pathogen during commercial Octopus vulgaris ICES Journal of Marine Science, 2007; 64: 1743-1748.
- Mladineo I, Bocina I. Extraintestinal gamogony of Aggregata octopiana in the reared common octopus (Octopus vulgaris) (Cephalopoda: Octopodidae). Journal of Invertebrate Pathology, 2007; 96: 261-264.
- Newton AL, Smolowitz R. Invertebrates. In: Pathology of Wildlife and Zoo Animals. Cambridge, MA: Elsevier. 2018: 1043.
- Noga EJ. Fish Disease Diagnosis and Treatment. 2nd Ames, IO: Wiley Blackwell. 2010; 148-150.
- Poynton SL, Reimschuessel R, Stoskopf MK. Aggregata dobelli Sp. and Aggregata millerorum N. Sp. (Apicomplexa: Aggregatidae) from Two Species of Octopus (Mollusca: Octopodidae) from the Eastern North Pacific Ocean. Journal of Protozoology, 1992; 39(1): 248-256.
- Seeley KE, Clayton LA, Hadfield CA, et al. Retrospective review of mortality in giant Pacific octopus (Enteroctopus dolfeini).