CASE 3: T18-09330 (4135870-00)
Signalment: 5-week-old pig (Sus scrofa domesticus)
History: The pig showed weight loss, and lethargy. Unthriftiness was also observed in other animals in the group. The pig was euthanized, and the carcass was received for examination.
Gross Pathology:
There was no grossly visible lesion on external examination. The carcass was found in a fair nutritional condition. A focal skin abrasion was observed on the head. Upon opening the carcass, it was noted that lungs were adhered to the pericardium and the parietal pleura with threads and mats of fibrin. The pericardium also was adhered to the epicardium with thick fibrin mat. Similar thin fibrin threads were observed on the serosal surfaces of the abdominal viscera. Stomach was empty. Liver was congested.
Laboratory results: Culture on lung tissues yielded colonies of Haemophlius parasuis. No bacterial growth was observed on cultures from liver, spleen and kidneys. Fluorescent antibody test for Porcine Reproductive and Respiratory Syndrome was negative.
Microscopic description:
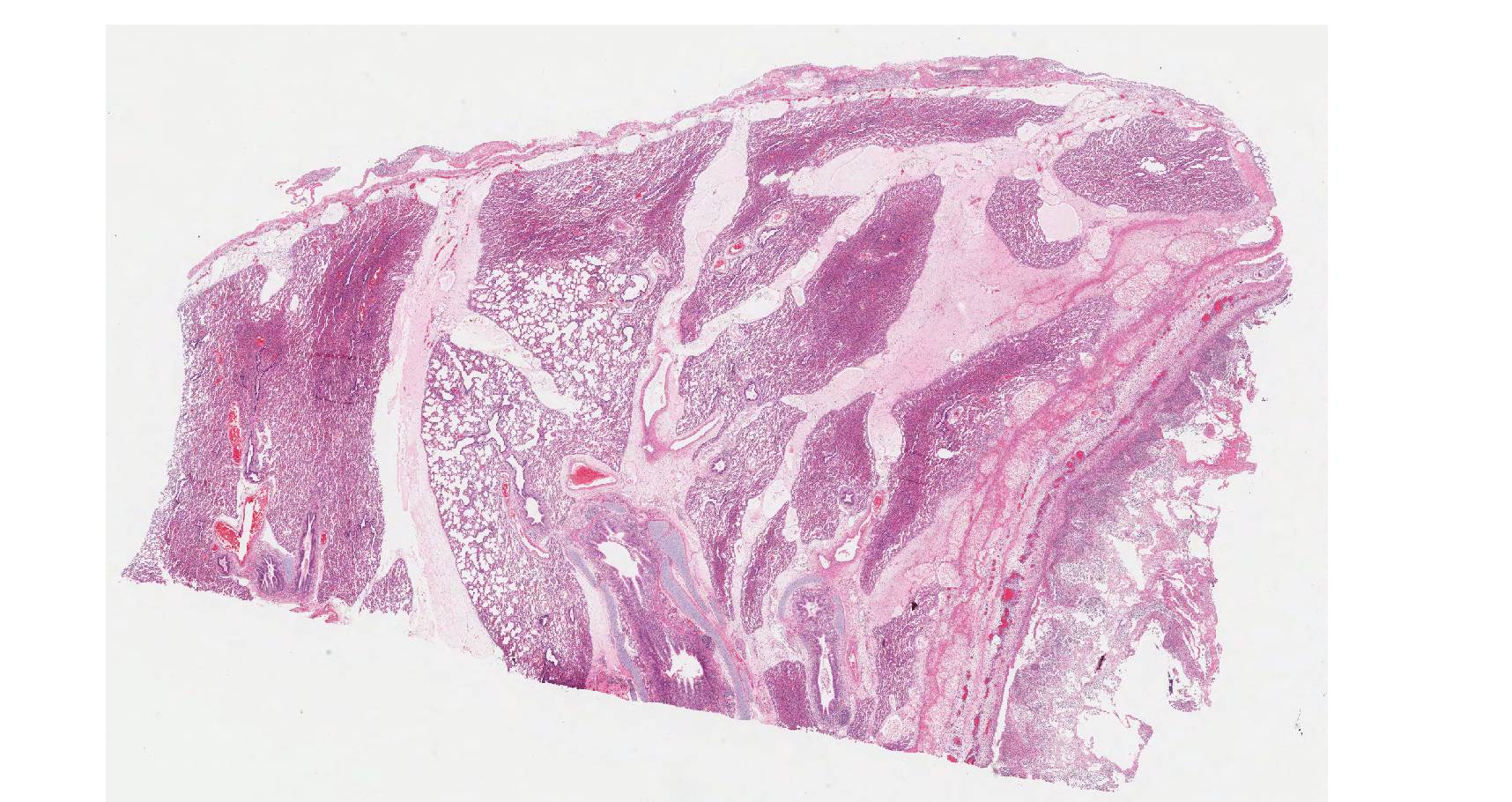
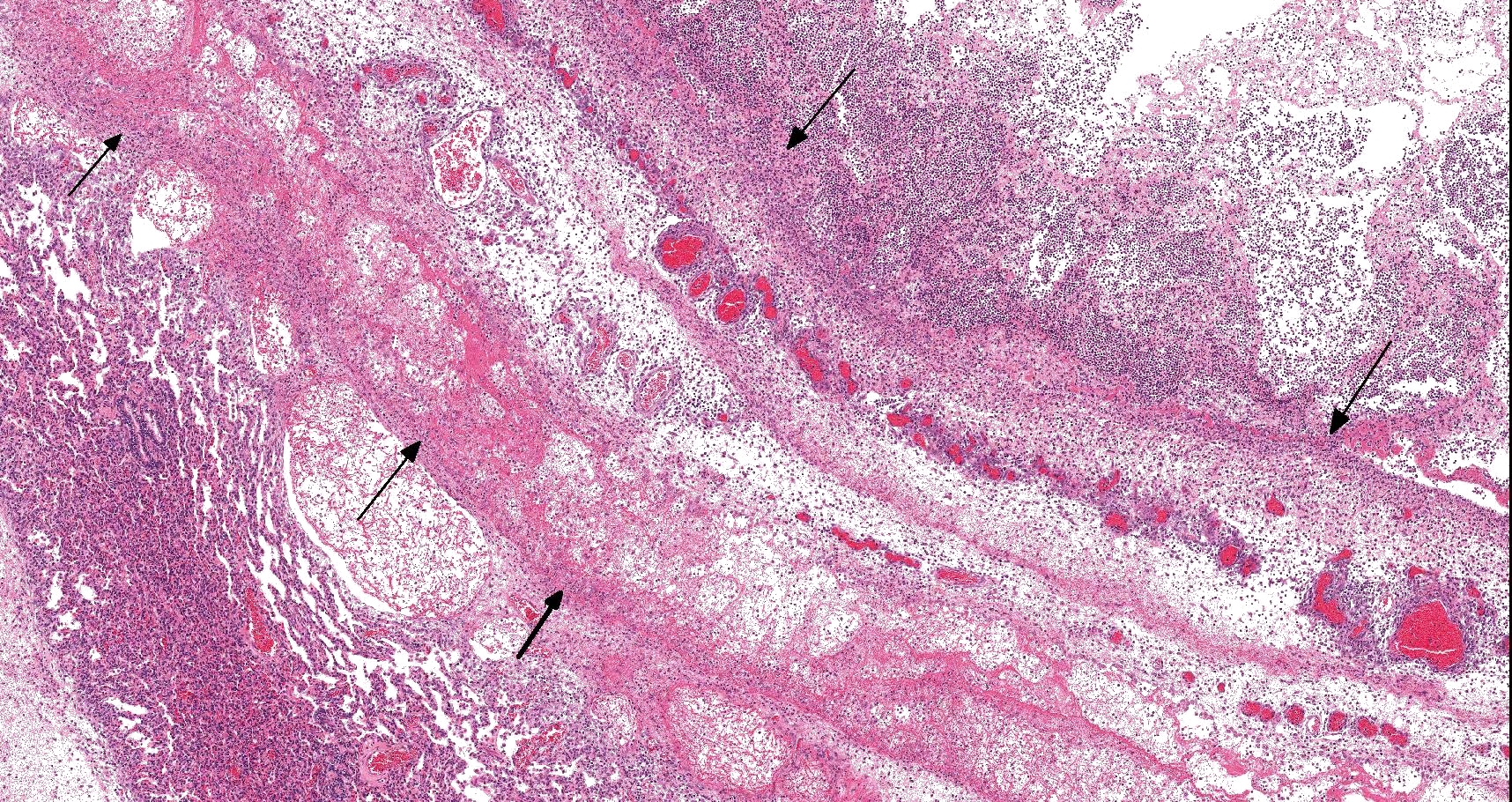
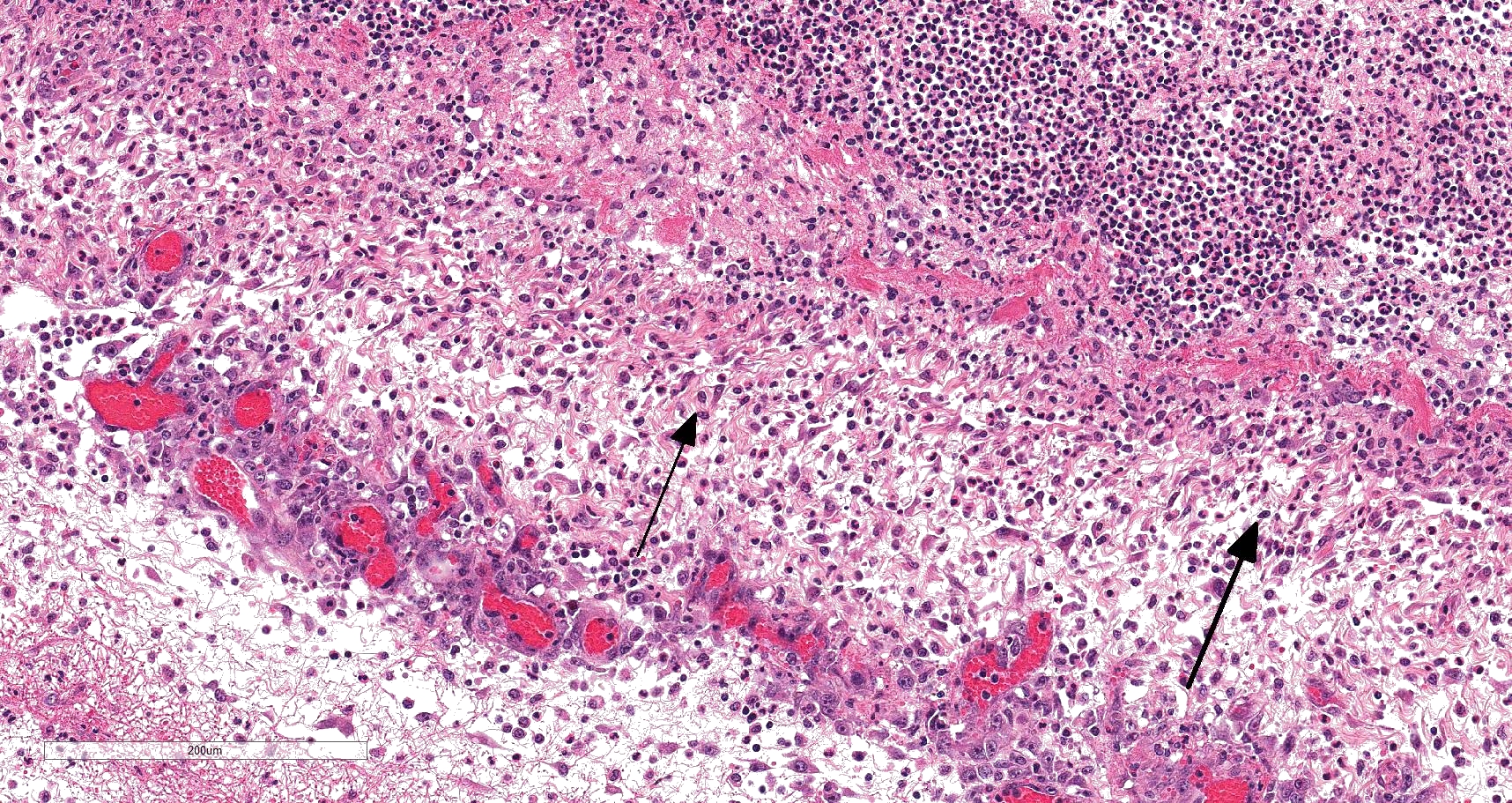
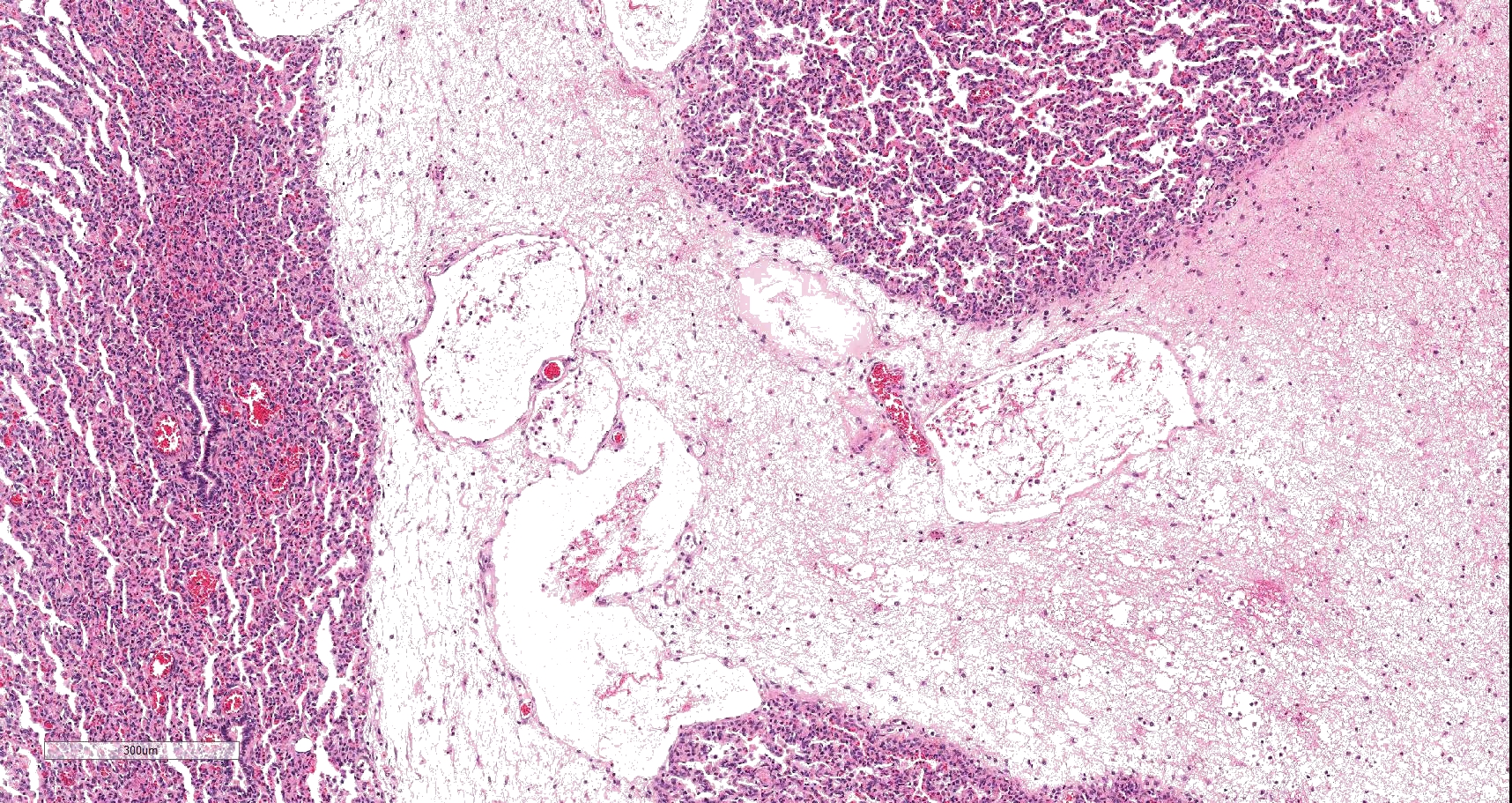
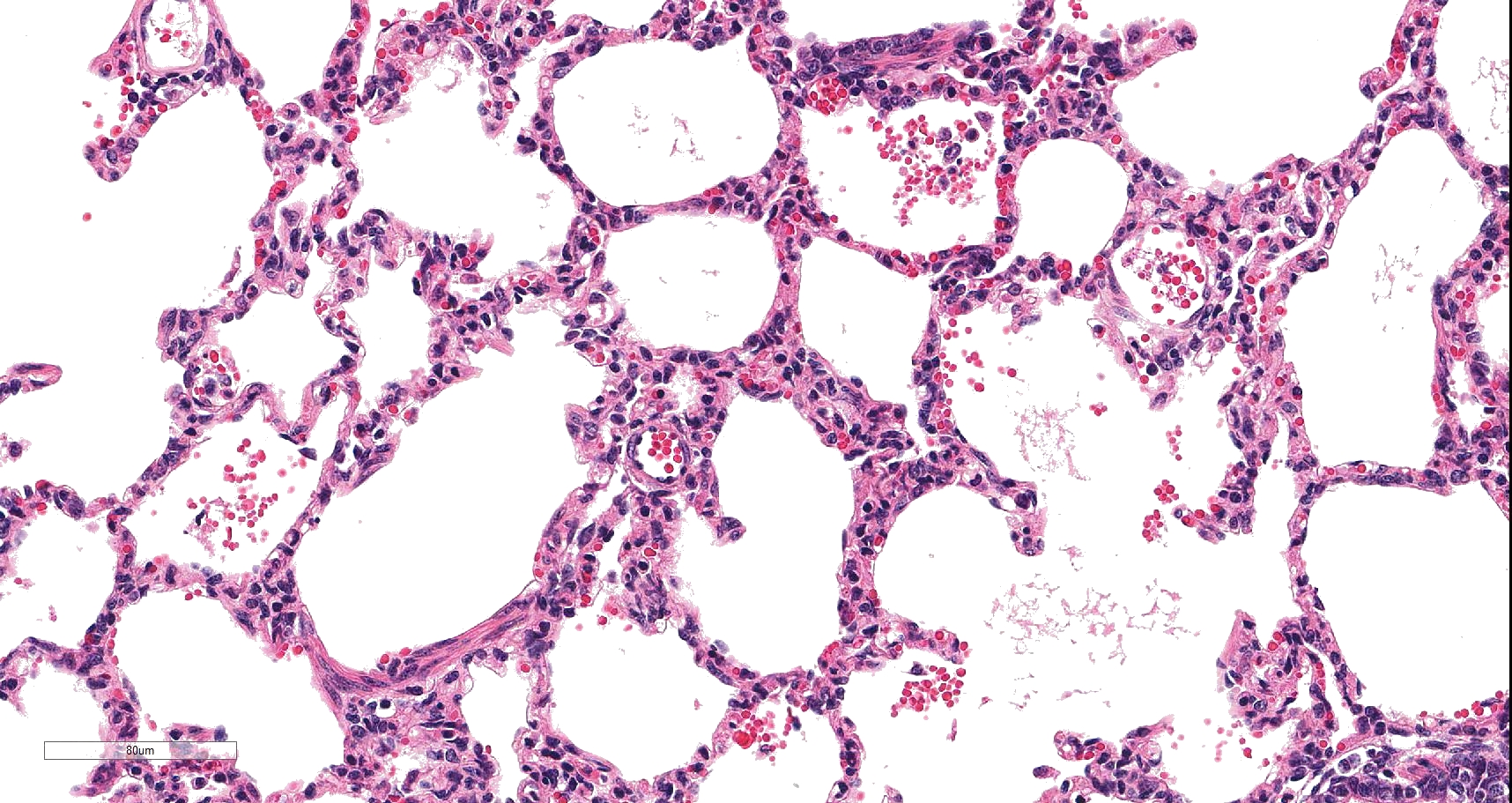
On histology, lesions vary from section to section. Abundant dead and degenerate neutrophils, necrotic debris, edema and fibrin expanded and disrupted the visceral pleura and occasionally extended to the subjacent pulmonary parenchyma. Multifocal aggregates of bacteria were observed in the necrotic areas. The remaining pulmonary tissue had diffusely collapsed alveoli (atelectasis). Similar dead and degenerate neutrophils, fibrin and necrotic debris expanded the pericardium, the epicardium, and partly extended to myocardium, and were also observed on the serosa of small intestines (slides not included). Large numbers of dead and degenerate neutrophils infiltrated multiple sections of reactive lymph nodes with moderate follicular hyperplasia. The findings in the other examined tissues were within normal limits.
Contributor's morphologic diagnosis:
- Severe, fibrinonecrotic and suppurative pleuritis/pleuropneumonia with bacteria
- Severe, fibrinonecrotic and suppurative epicarditis, pericarditis and myocarditis (slide not submitted)
- Moderate multifocal abdominal visceral serositis/peritonitis (slide not submitted).
Contributor's comment:
The grossly and microscopically observed lesions including severe, diffuse, fibrinous pleuritis, fibrinous pericarditis, and mild fibrinous peritonitis are consistent with polyserositis. Polyserositis is inflammation of serous membranes of the pleura, pericardium, and peritoneum7 and in pigs is often associated with infection due to Haemophlius parasuis, the cause of Glässer's disease. The disease is characterized by polyserositis, polyarthritis, and fibrinous meningitis. It is a major bacterial infection with worldwide distribution that has caused considerable economic losses even in high health status farms in the pig industry due to increase in mortality and morbidity. The bacterium, H. parasuis is a gram-negative, small, rod-shaped, pleomorphic, non-hemolytic, non-motile, nicotinamide adenine dinucleotide (NAD)-dependent and microaerophile bacterium that belongs to the genus Haemophilus of the family Pasteurellaceae.6,9,12 The bacterium colonizes the upper respiratory tract of healthy pigs and, under certain circumstances, some strains are able to invade the host and cause severe lesions. Transmission of infection occurs by aerosols and suspended particles in the air and in susceptible animals it causes Glässer's disease, pneumonia and sudden death in pigs.9 Fifteen serotypes of H. parasuis have been identified4 and individual serovars differ in virulence ranging from highly virulent to nonvirulent, and considerable differences in virulence also exist within each serovar.4,6 Virulent strains can be microorganisms secondary to pneumonia, cause septicemia without polyserositis or Glässer's disease characterized by polyserositis, pericarditis, arthritis and meningitis.6
In pathogenic bacteria, virulence factors play a critical role in pathogenesis. Factors involved in the virulence and pathogenicity of H. parasuis that enable some strains to cause a clinical disease remain largely unclear. Acute infections are occasional, and the clinical disease particularly affects young animals exposed to stress. The immune status of the host is a determinant factor in the pathogenic potential of H. parasuis infection4,6,12 and as other members of Pasteurellaceae family, H. parasuis can avoid phagocytosis. Nowadays, the increase in the occurrence of Glässer's disease is being more associated to the current practices in animal production and with the emergence of immunosuppressive viruses.9
Clinical signs of acute and chronic disease are highly variable likely due to variation in the virulence of different strains and the immune status of the host.6,12 Glässer's disease occurs sporadically in young pigs due to stress factors. Three clinical forms of infection due to H. parasuis are recognized. The lesions observed in the first form are fibrinopurulent exudate on serosal surface (polyserositis) of synovium, pericardium, peritoneum, pleura and meninges; and in the second form septicemia without polyserositis, sub-capsular kidney bleeding and sudden death are observed; while in the third form, H. parasuis can cause pneumonia and be isolated as primary or secondary agent in infections with Porcine Circovirosis (PCV2) and Porcine Reproductive and Respiratory Syndrome (PRRS).9
Diagnosis of Glässer's disease is based on clinical signs, presence of lesions at necropsy, and bacteriologic culture. Serological diagnosis of H. parasuis is inconsistent and inaccurate. Nowadays, the development of PCR with more sensitivity and specificity has improved laboratory diagnosis of infection with H. parasuis. The development of molecular techniques has improved identification of virulence factors, differentiated and genotyped strains, defined the true prevalence of systemic infection, and helped to better understand infection and diseases mechanisms.9 Various bacteria may cause similar lesions and should be considered in the differential diagnoses. A significant association between the detection of H. parasuis and M. hyorhinis in serosal swabs taken from pigs with polyserositis has been documented. Therefore, infection with Mycoplasma hyorhinis must be considered as a differential diagnosis since it can cause polyserositis, arthritis, and pneumonia, clinically indistinguishable from Glässer's disease.8 Polyserositis and meningitis were also reported associated with Escherichia coli infection in piglets. Although not a common cause of mortality in piglets, septicemic colibacillosis could cause high mortality in piglets and should be considered as a differential diagnosis in cases of polyserositis and meningitis.11
Effective prevention and control of H. parasuis have to be done following an epidemiological study in each herd.9 The disease caused by H. parasuis including polyserositis can be treated with antibiotics; however, oral or parenteral administration of very high doses of antibiotics is necessary as soon as possible after the manifestation of clinical signs.6,7 However, pigs with clinical signs such as growth retardation and a rough coat have a poor prognosis despite antibiotic therapy; and antibiotic treatment is usually unsuccessful because of the presence of the causative agent in the serous membranes.7
The ideal method to prevent H. parasuis infection is vaccination of sows and piglets combined with prophylactic antibiotic treatment of newborn piglets.4 Commercial or autogenous vaccines can be used in the immunoprophylaxis of pre-parturient sows and piglets after weaning.6 To prevent the herd from dissemination and infection, even in small farms, some practices such as early weaning and segregated production are recommended.9 The level of animal hygiene and animal husbandry are important factors for prevention of this disease.6 Some stressful practices such as weaning, transportation, and numerous sites of production may influence the epidemiology of H. parasuis within herds. Use of uniform age to weaning and minimizing stress factors that cause immunosuppression will help to prevent and control clinical disease.9
Contributing Institution:
The University of Georgia, College of Veterinary Medicine, Department of Pathology, Tifton Veterinary Diagnostic & Investigational Laboratory, Tifton, GA 31793 http://www.vet.uga.edu/dlab/tifton/index.php
JPC diagnosis:
Lung: Pleuropneumonia, fibrinosuppurative, diffuse, moderate to severe, with marked intralobular edema and fibrin.
JPC comment:
Karl Glässer first described a condition in pigs in Die Krankheiten des Schweines around 1906, which included a serofibrinous pleuritis, pericarditis, peritonitis, arthritis, and meningitis. However, the bacterium was not isolated until 1922 by Schermer and Ehrlich.6 Around the year 1912, there was a great deal of confusion about whether Hog Cholera was caused by a pair of bacteria (bacillus suipestifer and bacillus suisepticus) or a virus, there was increasing evidence of a viral etiology. However, at the time, there was a "Pectoral Form" of Hog Cholera that captured many features of what would become known as Glässer's disease. In Special Pathology and Therapeutics of the Diseases of Domestic Animals (1912), the pectoral form of Hog Cholera included a possibility of fibrinous or sero-fibrinous pleurisy and sometimes a similar pericarditis. Glässer had isolated a potentially novel bacillus from two spontaneously affected pigs which he felt might independently cause primary disease. The considerable overlap between disease presentations, the difficulty in identifying bacteria, and the shift to a viral etiology for Hog Cholera presented numerous challenges to researchers at the time.3
Significant resources have been allocated to research of Haemophilus parauis in the last decade, resulting in refinement of our understanding of the disease. More than 15 serotypes have been described around the world, with a number of identified genes encoding virulence factors such as sialyltransferase (lsgB), polysaccharide biosynthesis protein (capD), polysaccharide export proteins (wza), various glycosyltransferases (HPM-1370, HPM-1371, HPM-1372, HPM-1373), and virulence-associated trimeric autotransporters (vta1, vta2, vta3). A recent study correlated these genes encoding proposed virulence factors with the different serotypes found in Central Vietnam, showing a statistically significant correlation between the presence of the HPM-1371, HPM-1372, capD, and vta1 genes and the serotype being highly virulent. Conversely, there was a decreased probability of the HPM-1371 and vta1 genes being present in the moderately and non-virulent serotypes.10
Virulence-associated trimeric autotransporters (VtaA) are exposed surface proteins expressed by virulent serotypes and strains of H. parasuis and use a Type V secretion system. These autotransporters have an inner membrane domain, translocator domain, and a passenger domain. The passenger domains of all VtaAs have repeats and motifs characteristic of adhesins, hemagglutinins, invasins, and variable numbers of triple helix collagen-like repeats. In the case of the vta2 product, it was determined that this specific autotransporter likely allowed the bacteria to attach to proteins like collagen, mucin, and fibronectin, but did not contribute to cell adhesion or invasion.1
However, it has been shown that H. parasuis infection increases expression of TGF-B1, and that pre-treatment of porcine kidney cells (PK-15) in vitro inhibits invasion by H. parasuis. There was a correlation between TGF-B1 expression and expression of fibronectin (Fn) and a5 integrin (a5), suggesting that these are critical molecules that inhibit cellular invasion by H. parasuis. Interestingly, extracellular matrix molecules (e.g. fibronectin, a5 integrin) have been associated with cellular invasion in Streptococcus pneumoniae, group A Streptococcus pyogenes, Staphylococcus aureus, and Haemophilus influenza.5
Recent phylogenetic analysis of 70 genomes showed H. parasuis divided into two tightly grouped clades that more recently diverged than their closest relatives, Bibersteinia. The two clades have so recently diverged that there are few new mutations to distinguish the groups, and classification cannot be performed confidently. Through combined genetic analysis and morphologic differences from Haemophilus, this bacterium has been recently taxonomically classified and renamed as Glaesserella parasuis.2
References:
- Costa-Hurtado M, Garcia-Rodriguez L, Lopez-Serrano S, Aragon V. Haemophlus parasuis VtaA2 is involved in adhesion to extracellular proteins. Veterinary Research. 2019;50(1):69.
- Dickerman A, Bandara AB, Inzana TJ. Phylogenomic analysis of Haemophilus parasuis and proposed reclassification to Glaesserella parasuis, gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2020;70:180-186.
- Hutyra F, Marek J, eds. Special Pathology and Therapeutics of the Diseases of Domestic Animals, Vol. 1. Alexander Eger: Chicago, IL; 1912:257-285.
- Kielstein P, Rapp-Gabrielson VJ. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J Clin Microbiol. 1992; 30(4): 862-865.
- Li Y, Zhang Y, Xia Y, Shen Y, Zhang J. Haemophilus parasuis modulates cellular invasion via TGF-B1 signaling. Veterinary Microbiology. 2016;196:18-22.
- Nedbalcova K, Satran P, Jaglic Z, et al. Haemophilus parasuis and Glässer?s disease in pigs: a review. Veterinarni Medicina, 51, 2006 (5): 168?179.
- Oh Y, Han K, Seo HW, et al. Program of vaccination and antibiotic treatment to control polyserositis caused by Haemophilus parasuis under field conditions. Can J Vet Res. 2013; 77(3):183-90.
- Palzer A, Haedke K, Heinritzi K, et al. Associations among Haemophilus parasuis, Mycoplasma hyorhinis, and porcine reproductive and respiratory syndrome virus infections in pigs with polyserositis. Can Vet J. 2015; 56 (3):285-7.
- Pereira D, Dalla Costa F, Ferroni L, et al. The challenges with Glässer?s disease in technified pig production. Aust J Vet Scien. 2017; 49(2), 63-69.
- Van CN, Thanh TVT, Zou G, et al. Characterization of serotypes and virulence genes of Haemophilus parasuis isolates from Central Vietnam. Veterinary Microbiology. 2019;230:117-122.
- Wilkie W. Polyserositis and Meningitis Associated with Escherichia coli Infection of Piglets Can Vet J. 1981; 22 (5): 171?173.
- Zhang B, Tang C, Liao M, et al. Update on the pathogenesis of Haemophilus parasuis infection and virulence factors. Vet Microbiol. 2014; 168 (1):1-7.