WSC 2023-2024, Conference 12, Case 3
Signalment:
Juvenile intact female Black Angus calf (Bos taurus)
History:
Seven weaned calves had a history of rapidly progressive neurologic signs, blindness, recumbency, and death. No obvious ocular lesions were appreciated clinically, and animals were unresponsive to dexamethasone and thiamine administration.
Gross Pathology:
A 200 kg calf had mild postmortem autolysis. The anterior chambers of the right and left eyes contained a moderate amount of fibrin and opaque fluid (hypopyon). Within the cerebral white matter, there was a focally extensive area characterized by numerous, up to 2 mm diameter, dark red to brown foci (infarcts). Additional gross lesions included a severe mural and valvular endocarditis of the right atrium and ventricle, fibrinosuppurative polyarthritis, suppurative bronchopneumonia, and fibrinosuppurative bilateral otitis media.
Laboratory Results:
Aerobic culture of synovial fluid, brain, and meningeal swab: No growth detected.
Mycoplasma bovis PCR, lung: Positive.
Mycoplasma bovis PCR, synovial fluid: Negative.
Histophilus somni PCR, brain: Positive.
Histophilus somni PCR, lung: Positive.
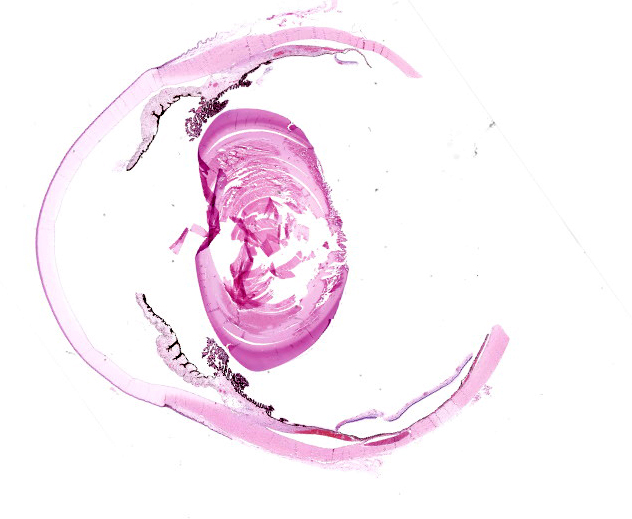
Microscopic Description:
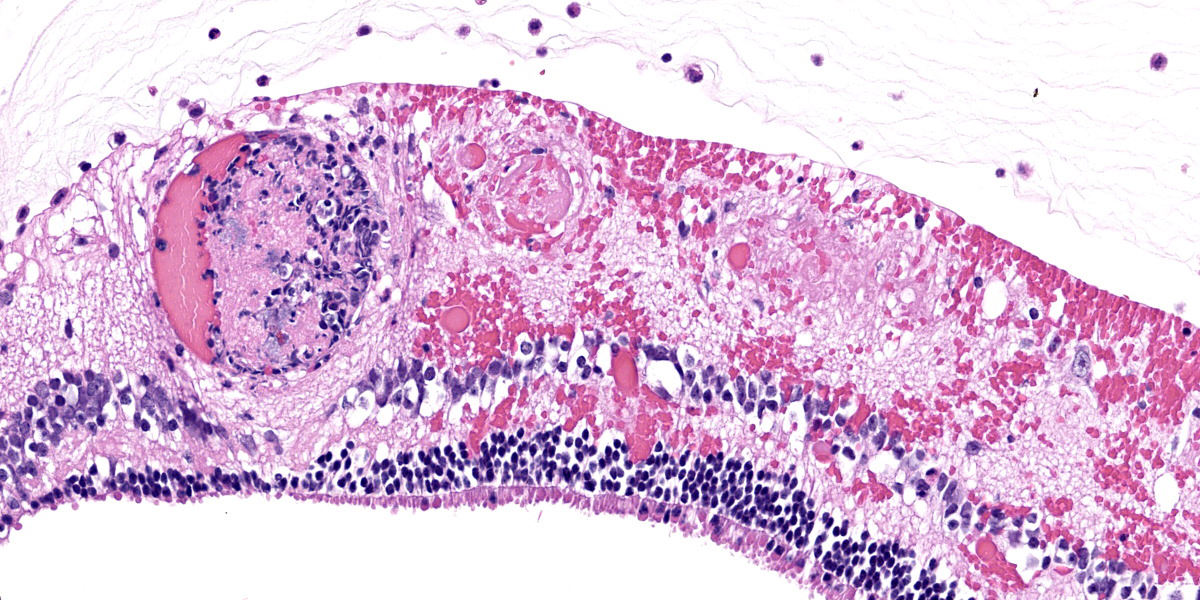
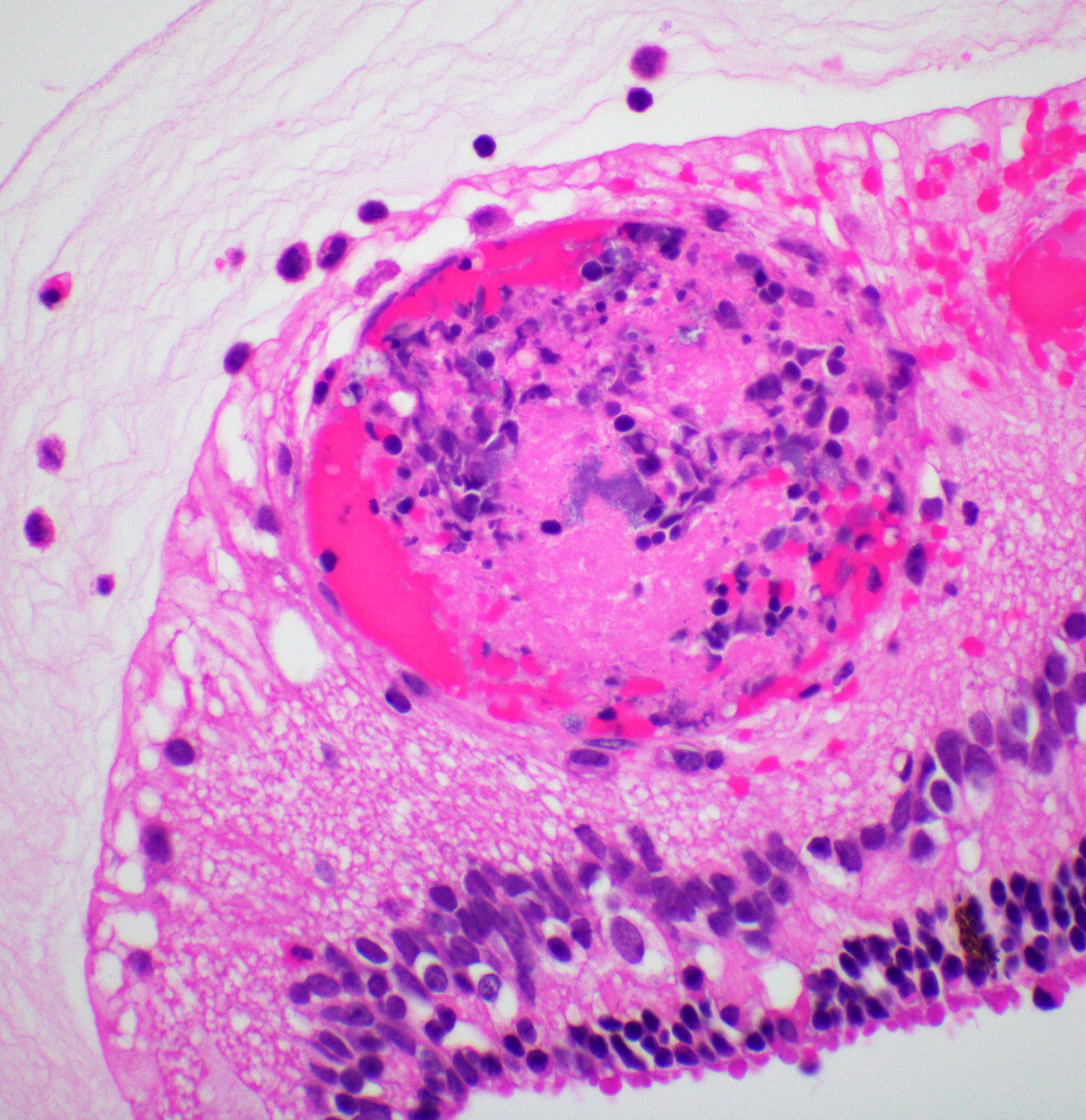
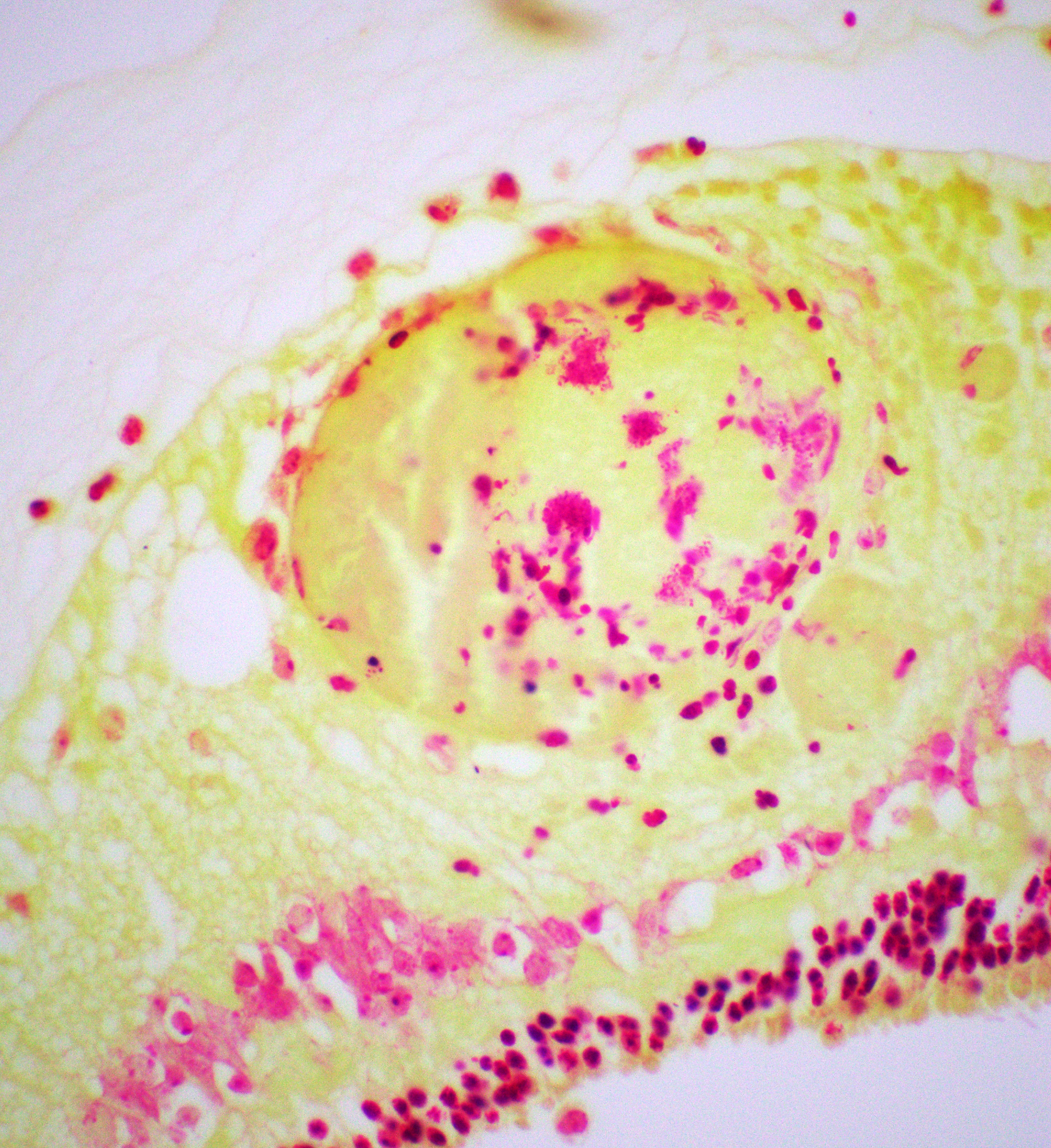
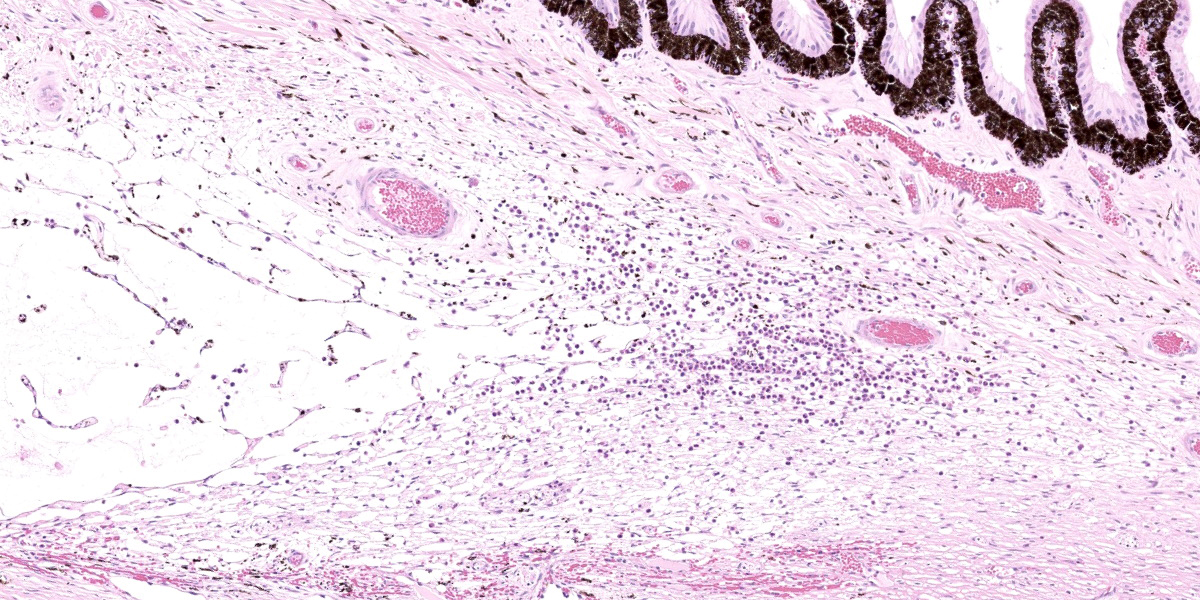
Right eye: Frequently, small and medium-caliber blood vessels within the retina, choroid, anterior uvea, and optic nerve (not present in the section) are expanded with partial to complete occlusion of vascular lumina by fibrin thrombi containing small numbers of degenerate neutrophils and small numbers of gram-negative coccobacilli. The walls of affected blood vessels are transmurally infiltrated and effaced by moderate numbers of viable and degenerate neutrophils and small amounts of fragmented cellular and nuclear debris (necrotizing vasculitis). Frequently, retinal tissue surrounding the most severely affected vessels is edematous and contains infiltrates of small to large numbers of degenerate neutrophils admixed with hemorrhage, necrotic cellular debris, and fibrin (ischemic necrosis). The subretinal space and vitreous contain small numbers of neutrophils admixed with hemorrhage and large amounts of loose fibrin. Free floating within the anterior chamber and disrupting and infiltrating the ciliary body at the iridocorneal angle are small to moderate amounts of fibrin admixed with small numbers of viable and degenerate neutrophils.
Contributor’s Morphologic Diagnoses:
- Right eye: Severe, multifocal to coalescing, acute necrohemorrhagic chorioretinitis and anterior uveitis with necrotizing fibrinoid vasculitis, fibrin thrombi, and intralesional gram-negative coccobacilli.
- Right eye: Moderate, multifocal to locally extensive, acute to subacute hypopyon.
Contributor’s Comment:
Histophilus somni (formerly Haemophilus somnus) is an economically significant disease of cattle and, to a lesser extent, sheep. Part of the Bovine Respiratory Disease Complex, it is associated with acute, fulminant shipping fever pneumonia primarily characterized by a fibrinous and suppurative bronchopneumonia with leukocyte necrosis, bronchiolar epithelial attenuation, and intravascular thrombi. While more commonly associated with pneumonia caused by Mannheimia haemolytica, oat cells are a commonly appreciated histologic feature.4,9
H. somni bacteremia, often a sequela to pneumonia, is reported to cause numerous and often severe changes in several tissues across multiple organ systems – termed the H. somni disease complex.5,8,12,13 The most well-known manifestation of this syndrome is thrombotic meningoencephalitis (TME) characterized grossly by multiple foci of hemorrhage and necrosis throughout the brain and spinal cord, and histologically by necrotizing vasculitis with intravascular
thrombosis and ischemic necrosis of dependent tissues.2,10 Other common disease entities secondary to bacteremia include otitis externa, laryngitis/tracheitis, myocarditis, metritis causing abortion or infertility, polyarthritis, mastitis, epididymitis, ampullitis, orchitis, and conjunctivitis.2 The hallmark histologic lesion is vasculitis with secondary thrombosis, and colonies of gram-negative coccobacilli are commonly observed within vascular thrombi, affected blood vessels, and infarcted tissues most commonly affecting the central nervous system and retina, as observed in this case.2
Considered part of the normal flora of the male and female genital tracts and the nasal cavity, several mechanisms of transmission have been proposed.5,8,12,13 Calves are suspected to become infected within the first few months of life via aerosolized urine and genital discharges. As with other pathogens of the bovine respiratory disease complex, infection is usually preceded by stressful events such as weaning, routine cattle processing, or shipping.3,4,8,9,13 Synergism with concurrent viral infections, particularly Bovine Respiratory Syncytial Virus, in further facilitating lung damage, pneumonia, and bacteremia has been documented.1,3 In cases of pneumonia, several virulence factors facilitating disease have been proposed including immunoglobulin Fc binding proteins (IgBPs) to resist serum and complement mediated killing; transferrin-binding proteins used in iron acquisition; biofilm formation; free oxygen-radical inhibition; and various other mechanisms of intracellular survival and immune evasion.2,4,5,8,9,12,13 While evasion of bovine neutrophils and macrophages has been inconsistently reported as a potential virulence factor, the innate immune system (primarily via extracellular traps produced by neutrophils, macrophages, mast cells, and eosinophils) has been observed to successfully restrict the growth of H. somni in several anatomic locations, including the respiratory system.8
In cases of vasculocentric pneumonia or vasculitis secondary to bacteremia, surface lipo-oligosaccharide (LOS) has been proposed as a significant virulence factor. LOS has been demonstrated in vitro to induce endothelial cell apoptosis via activation of caspase 3, triggering the production of reactive oxygen species and nitrogen intermediates.2,5,12,13 Another pathway suggests LOS triggers platelet activation leading to endothelial apoptosis via caspases 8 and 9, endothelial cell cytokine production and adhesion molecule expansion, and promotion of endothelial cell production of reactive oxygen species which further enhances endothelial apoptosis.2 LOS has been demonstrated to undergo
phase and antigenic variation and sialylation contributing to immune resistance.5,13 It has been proposed that predisposed sites of infarctive lesions and vasculitis include those in which there are dramatic changes in blood vessel diameter or flow patterns that increase the likelihood of endothelial or platelet activation.10
In the present case, necrotizing and fibrinoid vasculitis with thrombosis and tissue infarction was observed within the cerebrum, cerebellum, brainstem, right eye, and left ventricle and papillary muscle of the heart. In addition, there was a severe fibrinosuppurative bronchopneumonia, right ventricular endocarditis, fibrinosuppurative otitis media, and multifocal fibrinosuppurative polyarthritis. Samples of lung and brain were positive for H. somni on PCR. Mycoplasma bovis was an important differential diagnosis in this case, and PCR testing was positive for M. bovis in the lung but negative in the synovial fluid of the joints.
Contributing Institution:
Department of Veterinary Pathobiology
Oklahoma State University
Stillwater, OK 74078 USA
https://vetmed.okstate.edu/veterinary-pathobiology/index.html
JPC Diagnosis:
Eye, retina and uvea: Vasculitis, necrotizing, multifocal, with fibrin thrombi, coccobacilli, and mild neutrophilic endophthalmitis.
JPC Comment:
Ocular histophilosis is a less common presentation of thrombotic meningoencephalitis (TME) in cattle; when present, however, the lesions are identical to the common, well-described lesions found in the brain of affected animals.15 Ocular histophilosis commonly presents with suppurative thrombotic lesions in the choroid and the retina, often with intravascular bacterial colonies visible on H&E.6,15 Ocular histophilosis can also present with retinitis as the sole ocular lesion, a histologic presentation shared with toxoplasmosis and infections with neurotropic viruses.15 The presence of ocular lesions is useful as an aid in clinical diagnosis of TME and is estimated to occur in 30-50% of animals with the septicemic form of the disease.15
The contributor provides an excellent overview of the typical disease syndromes of histophilosis and of the virulence factors which cause its characteristic lesions. In addition to LOS, the key virulence factor discussed above, H. somni appears able to form biofilms on the endothelial surfaces of the microvasculature, particularly in the heart and
the brain.11 Biofilms are collections of bacteria enclosed in a matrix composed of extracellular polysaccharides, proteins, and nuclei acids. The matrix allows cells to adhere to each other and/or other surfaces and allows for metabolic cooperation among the colony.11 The matrix also provides protection from antibacterial agents, with bacteria within biofilms being less susceptible to antimicrobial therapy.11 It is unknown whether biofilm formation occurs within ocular histophilosis, but biofilm formation and the subsequent concentration of endotheliotoxic virulence factors appears to be a significant contributor to pathogenesis in cardiac histophilosis and TME.11
Conference discussion initially revolved around differential diagnoses, with the moderator gently prodding residents to recall Chlamydia pecorum, which can cause many of the same clinical signs and histologic lesions as H. somni. Chlamydia pecorum can be cound in aborted fetuses, calves, and cows, and can cause meningoencephalitis, thrombosis, vasculitis, pericarditis, and polyserositis, with intracytoplasmic bacteria in endothelial and mononuclear cells visible on histology.14
Dr. Neto discussed the various manifestations of histophilosis and noted that a negative culture does not necessarily rule out histophilosis. A recent study found that culture of Histophilus somni from infection-confirmed tissue is often unsuccessful, making PCR the preferred diagnostic modality.7 Finally, Dr. Neto discussed the very nature of the thrombus itself, providing examples of fibrin-rich thombi, with the expected PTAH stain positivity, and platelet-rich thrombi, which did not stain with PTAH. Immunohistochemical staining with CD61, which forms part of the platelet fibrinogen receptor glycoprotein IIb/IIIa, can be used to identify these platelet rich, PTAH-negative thrombi in formalin-fixed tissues.
References:
- Agnes JT, Zekarias B, Shao M, Anderson ML, Gershwin LJ, Corbeil LB. Bovine respiratory syncytial virus and Histophilus somni interaction at the alveolar barrier. Infect Immun. 2013; 81:2592-2597.
- Cantile C, Youssef S. Nervous System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed., vol 1. Saunders Elsevier; 2016:364-365.
- Caswell JL. Failure of respiratory defenses in the pathogenesis of bacterial pneumonia of cattle. Vet Pathol. 2014;51: 393-409.
- Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed., vol 2. Saunders Elsevier;2016:494, 544-546.
- Corbeil LB. Histophilus somni host-parasite relationship. Anim Health Res Rev. 2007;8:151-160.
- Dukes TW. The ocular lesions of thromboembolic meningoencephalitis (TEME) in cattle. Can Vet Jour. 1971; 12(9):180-182.
- Headley SA, Pereira AHT, Balbo LC, et al. Histophilus somni-associated syndromes in sheep from Southern Brazil. Braz J Microbiol. 2018;49(3):591-600.
- Hellenbrand KM, Forsythe KM, Rivera-Rivas JJ, Czuprynski CJ, Aulik NA. Histophilus somni causes extracellular trap formation by bovine neutrophils and macrophages. Microb Pathog. 2012; 54: 67-75.
- Lopez A, Martinson SA. Respiratory System, Mediastinum, and Pleurae. In: Zachary JF, ed. Pathologic Basis of Veterinary Diseases. 6th ed. Elsevier; 2017:530-531.
- Miller AD, Zachary JF. Nervous System. In: Zachary JF, ed. Pathologic Basis of Veterinary Diseases. 6th ed. Elsevier; 2017:882.
- O’Toole D, Hunter R, Zekarias B, et al. Effect of Histophilus somni on heart and brain microvascular endothelial cells. Vet Pathol. 2017;54(4):629-639.
- Sandal I, Inzana TJ. A genomic window into the virulence of Histophilus somni. Trends Microbiol. 2010; 18:90-99.
- Siddaramppa S, Inzana TJ. Haemophilus somnus virulence factors and resistance to host immunity. Anim Health Res Rev. 2004; 5:79-93.
- Struthers JD, Lim A, Ferguson S, et al. Meningoencephalitis, vasculitis, and abortions caused by Chlamydia pecorum in a herd of cattle. Vet Pathol. 2021;58(3):549-557.
- Wilcock BP, Njaa BL. Special Senses. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed., vol 1. Saunders Elsevier;2016:474.
- Zachary JF. Mechanisms of Microbial Infections. In: Zachary JF, ed. Pathologic Basis of Veterinary Diseases. 6th ed. Elsevier;2017:189.