Signalment:
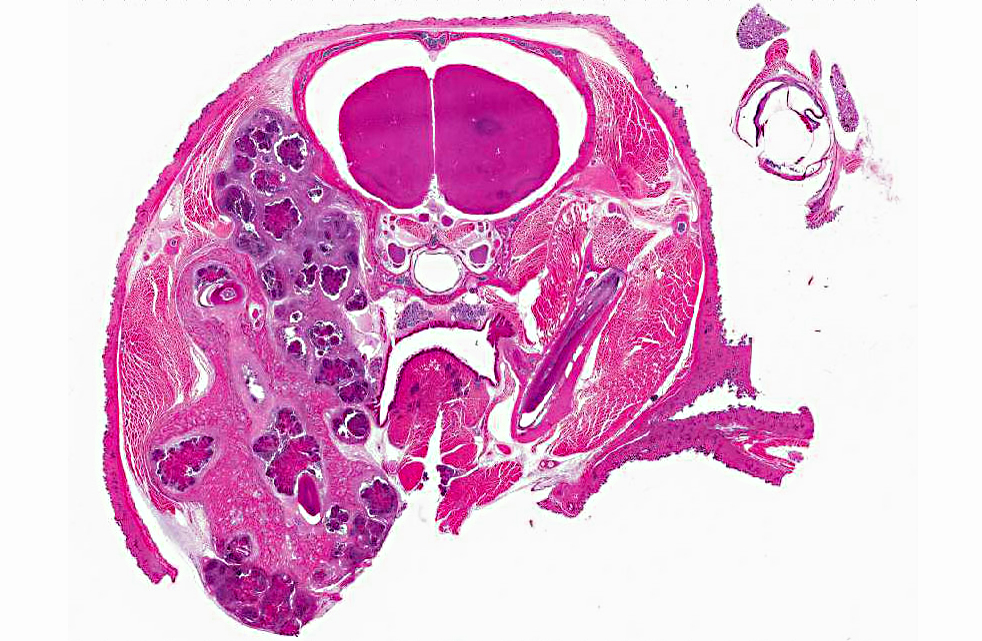
Gross Description:
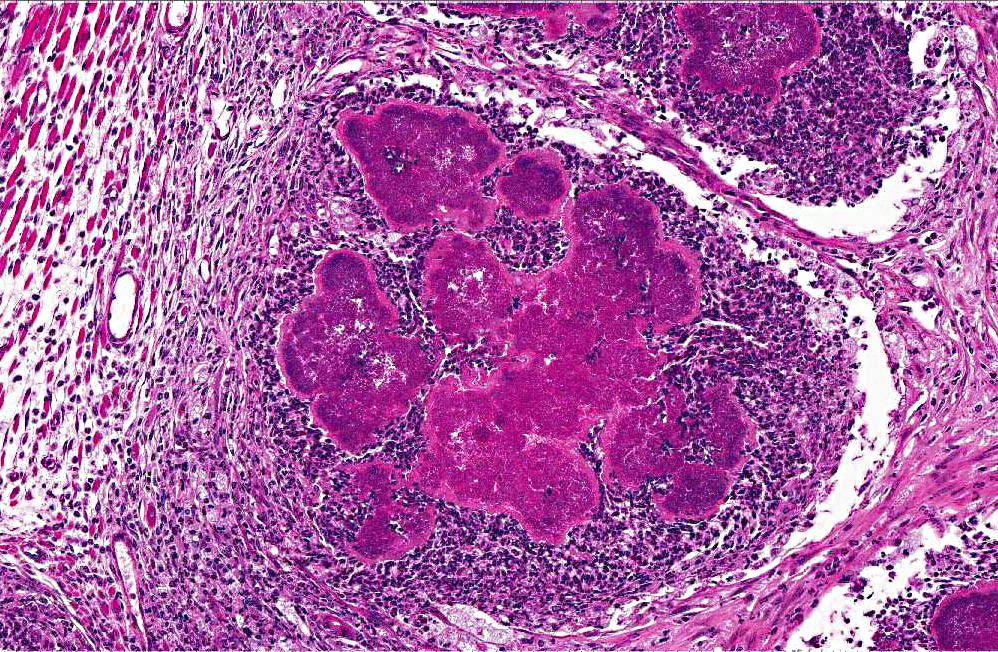
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
In addition to staphylococci (S. aureus, S. hominus, S. xylosus), other bacterial agents have also been isolated from cases of botryomycosis in rodents, including, Streptococcus intermedius, Pseudomonas aeruginosa, Proteus spp., Escherichia coli and Nocardia asteroides.(1,3,4,8,11) In other animals and in people however, Splendore-Hoeppli material has been reported to arise in association with infections due to fungi and other parasites, and has also been known to surround biologically inert substances such as suture material.(1,3,4,5,7,12)
Although the exact nature of this reaction is unknown, it is considered to be a localized immunological response to an antigen-antibody precipitate related to fungi, parasites, bacteria or inert materials.(5) The characteristic formation of the Splendore-Hoeppli reaction around infectious agents or biologically inert materials probably represents some attempt at containment of the agent on the part of the host. It likely prevents phagocytosis and intracellular killing of the injurious agent leading to chronicity of infection, so whether this degree of immune responsiveness is actually helpful or harmful remains to be determined.(5,6)
Similarly the nature of the intense eosinophilic nature of this material remains poorly understood, but is thought to be due to its composition of antigen-antibody complexes (immunoglobulins and major basic proteins) combined with cell debris from inflammatory cells (lymphocytes, plasma cells, eosinophils and macrophages) and fibrin.(5) Composition of the reaction likely depends on the type of causative agent, and one hypothesis is that Splendore-Hoeppli material may be derived from host leukocytes that aggregate in response to the bacteria, parasites or inert foreign materials that can initiate the reaction.(5,6)
Morphological mimickers of the Splendore-Hoeppli reaction are said to include flame figures (as seen in conditions such as feline eosinophilic granuloma complex, insect bites and drug eruption), tophaceous lesions of gout, asteroid bodies (as may be rarely seen in some granulomatous lesions in animals) and actinomycotic sulfur granules (the radiating structures of Splendore-Hoeppli reaction resemble these sulfur granules). The absence of central branching filaments in Splendore-Hoeppli reaction helps to distinguish this from true actinomycotic sulfur granules.(5)
Flame figures represent degranulated eosinophils that form aggregates of granular necrotic material surrounded by collagen, and these foci are often basophilic with peripheral macrophages.(5) Gout tophi appear as variably sized deposits of amorphous, amphophilic material with parallel, acicular clefts; however, these crystals often have a brownish color and are doubly refractile with polarized light.(5) Asteroid bodies are stellate eosinophilic inclusions seen within giant cells of granulomas occasionally.(5) This reaction is histologically opposite to that which is seen in the Splendore-Hoeppli reaction, i.e., a central stellate acellular region that is surrounded by inflammatory cells.(5)
JPC Diagnosis:
Conference Comment:
References:
2. Bridgeford EC, Fox JG, Nambiar PR, et al. Agammaglobulinemia and Staphylococcus aureus Botryomycosis in a Cohort of Related Sentinel Swiss Webster Mice. J Clin Microbiol. 2009;46(5):1881-1884.
3. EL van den Berk G, Noorduyn LA, van Ketel RJ, et al. A fatal pseudo-tumour: disseminated basidiobolomycosis. BMC Infectious Diseases. 2006;6:140.
4. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed. Philadelphia, PA: Elsevier; 2007:691.
5. Hussein MR. Mucocutaneous Splendore-Hoeppli phenomenon. J Cutan Pathol. 2008;35(11):979-988.
6. Mondino A, Blasi F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol. 2004;25(8):450-455.
7. Rodig SJ, Dorfman DM. Splendore-Hoeppli phenomenon. Arch Pathol Lab Med. 2001;125:1515-1516.
8. Schlossberg D, Pandey M, Reddy R. The Splendore-Hoeppli phenomenon in hepatic botryomycosis. J Clin Pathol. 1998;51:399-400.
9. Shapiro RL, et al. Urokinase-type plasminogen activator-deficient mice are predisposed to staphylococcal botryomycosis, pleuritis, and effacement of lymphoid follicles. Am J Path. 1997;2(1):359-369.Â
10. Spagnoli S, Reilly TJ, Calcutt MJ, et al. Subcutaneous botryomycosis due to Bibersteinia trehalosi in a Texas longhorn steer. Vet Pathol. 2012;49(5):775-778.
11. Weighardt H, Kaiser-Moore S, Vabulas RM, et al. Cutting edge: Myeloid differentiation factor 88 deficiency improves resistance against sepsis caused by polymicrobial infection. J Immnunol. 2002;169:28232827.
12. Zavasky D, Samowitz W, Loftus T, et al. Gastrointestinal zygomycotic infection caused by basidiobolus ranarum: Case report and review. Clin Infec Dis. 1999;28(6):1244-8.