CASE III: 17-145 (JPC 4117030-00)
Signalment:
2.5-year-old Hereford heifer, Bos taurus.
History:
42 of 187 grazing beef cattle died between June 28 and July 2017 in a farm in Florida department, Uruguay. Morbidity rate was 22.9%, mortality 22.5% and lethality 97.7% (the farmer indicated that one affected cow recovered). The herd had 42 cows, 70 2.5-year-old heifers, and 75 yearlings (heifers and steers). At the end of the outbreak dead animals included 3 cows (7.1%), 11 heifers (15.7%) and 28 yearlings (37.3%). Clinical signs included stupor or aggressiveness and drooling. The animals were found dead or died after a clinical course of up to three days. Numerous groups of insect larvae identified as Perreyia flavipes (sawfly) were found in the pasture.
Gross Pathology:
Hepatic changes included diffuse enhanced lobular pattern with yellowish edema expanding the gall bladder wall. Other necropsy findings were abdominal and pericardial effusion and petechiae and ecchymoses in the pericardium, peritoneum and pleura. Diffuse hemorrhages were observed on the capsular surface of the spleen. The omasal content was dry. Body fragments and heads of Perreyia sp. larvae were found in the content of the rumen and omasum.
Laboratory results:
Not applicable.
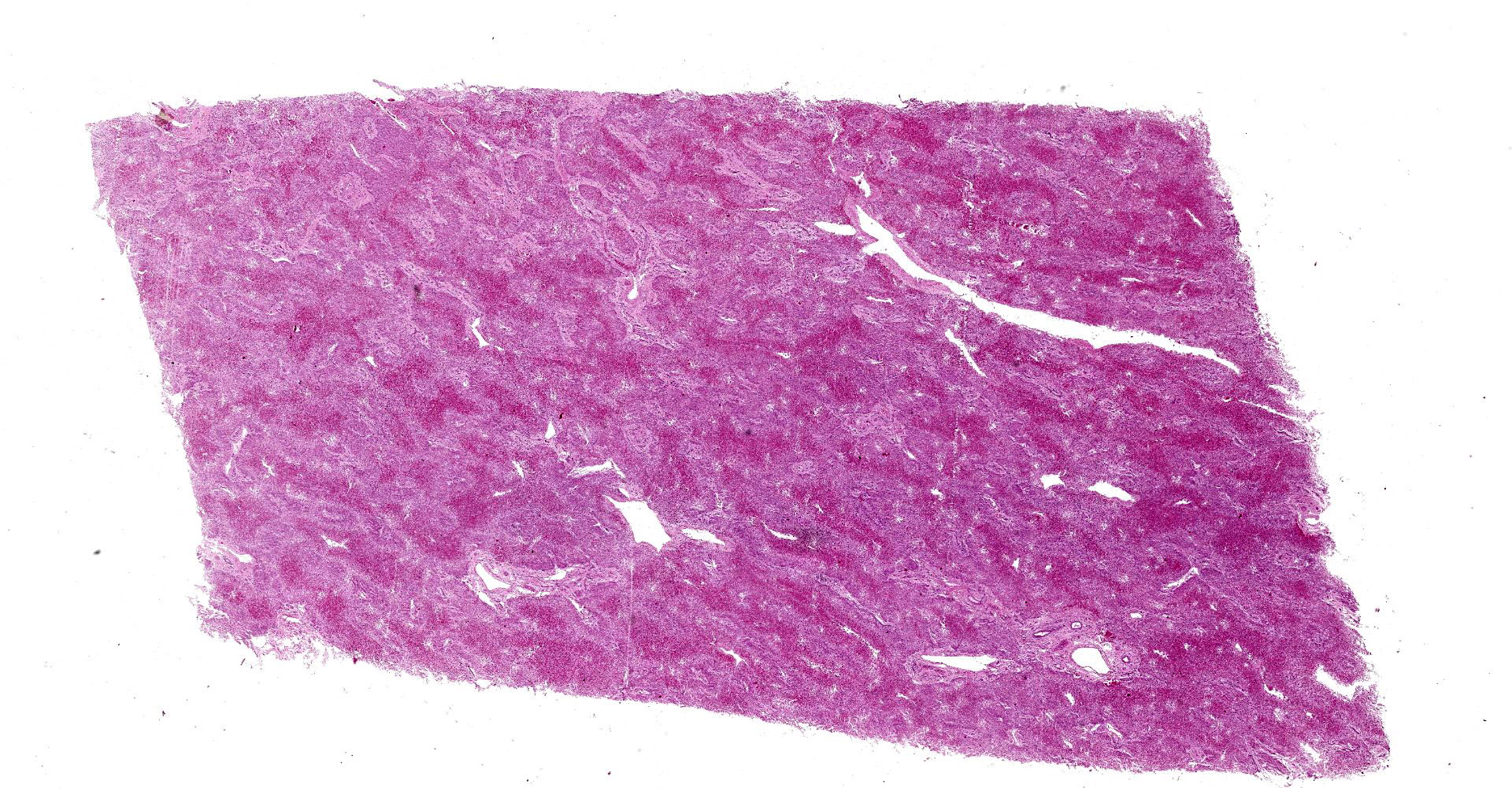
Microscopic Description:
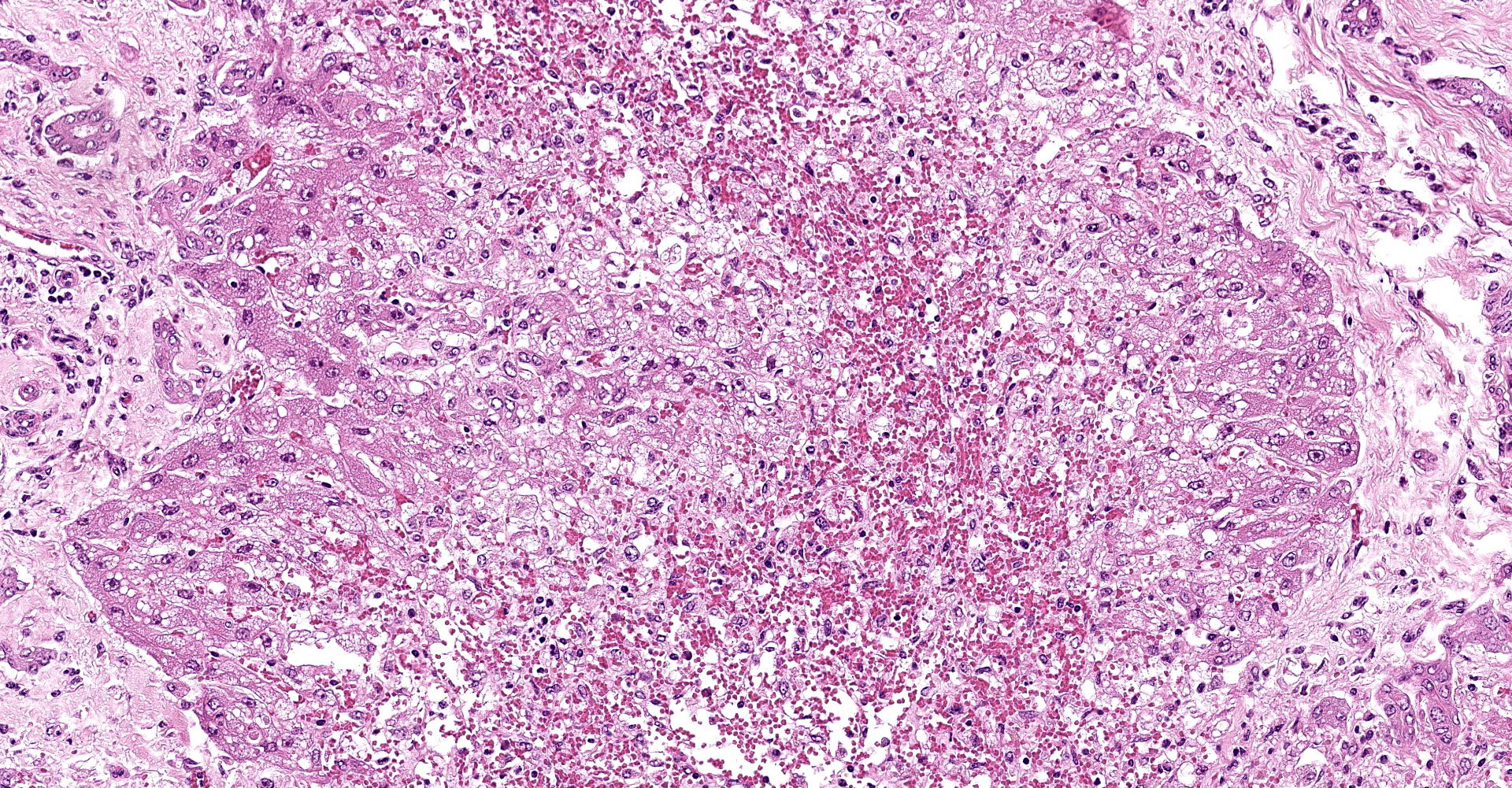
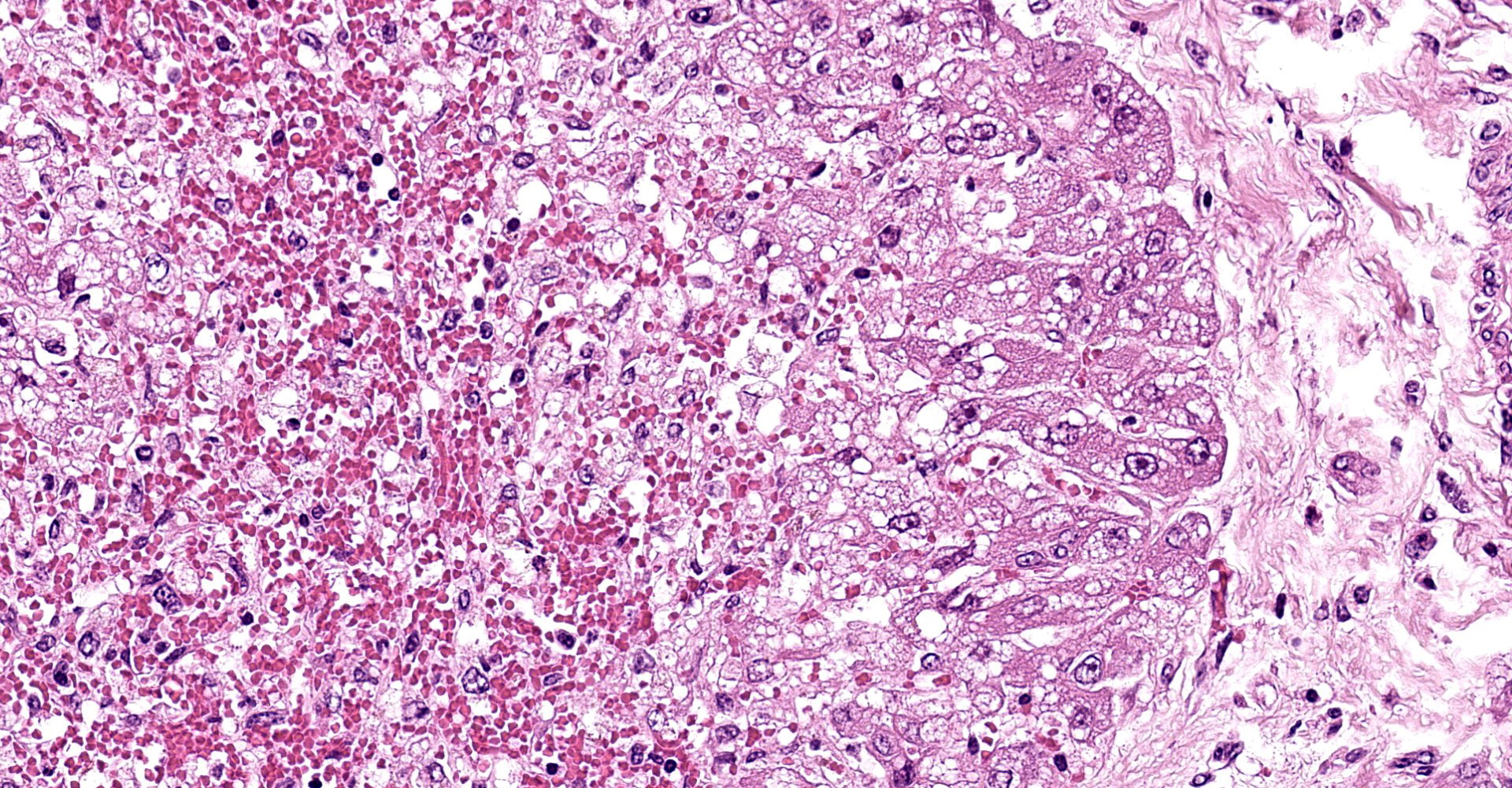
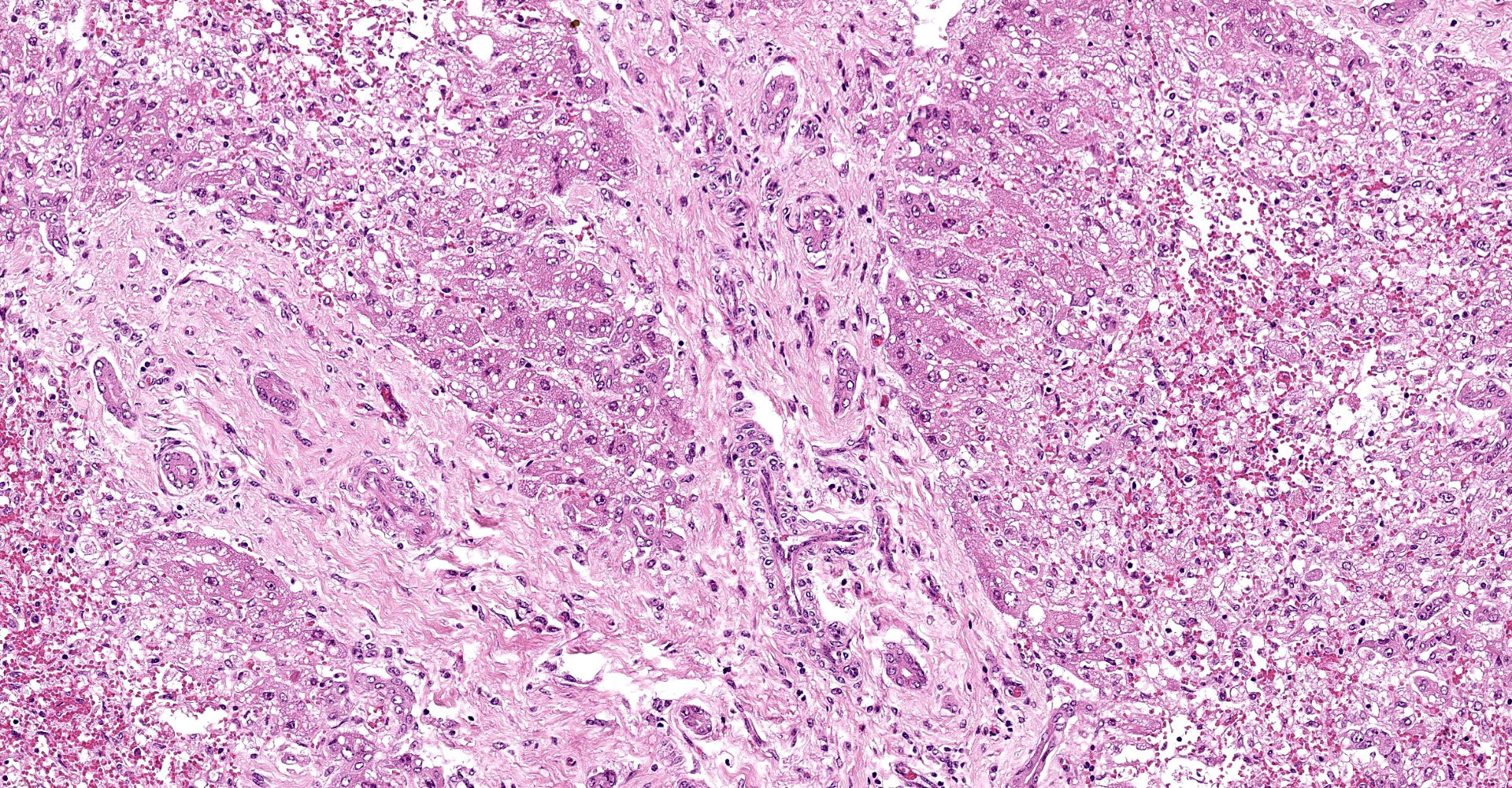
Liver: subgrossly, there is diffuse enhancement of the lobular pattern, the lobular histoarchitecture is effaced and hepatic lobules are collapsed. There is necrosis and loss of hepatocytes in the centrilobular and midzonal areas, and replacement by abundant extravasated erythrocytes (hemorrhage). Remaining hepatocytes are either rounded, swollen and detached from the hepatic cords, or have angular borders, pyknotic nucleus and shrunken hypereosinophilic cytoplasm (necrosis). In the periportal areas, remaining viable hepatocytes are swollen and vacuolated (hydropic and lipid degeneration), and there are occasional bi- or multi-nucleated hepatocytes (regeneration). There are increased numbers of histiocytes and neutrophils in the sinusoids, and Kupffer cell hypertrophy and hyperplasia. There is mild bile duct hyperplasia, and portal tracts are multifocally infiltrated by inflammatory cells, including histiocytes, lymphocytes, fewer neutrophils and eosinophils, and expanded by mature collagen bundles (fibrosis); similar changes extend to the hepatic capsule in some sections (not included in all slides).
Contributor 's
Morphologic Diagnosis:
1. Liver: severe diffuse acute centrilobular and midzonal
hepatocellular degeneration, necrosis, loss and hemorrhage, Bos taurus.
2. Liver: mild portal fibrosis and bile duct hyperplasia, with lymphohistiocytic portal hepatitis, Bos taurus.
Contributor 's Comment:
The diagnosis of Parreyia flavipes intoxication in this outbreak was based on the observation of severe acute centrilobular necrosis, the presence of large amounts of Perreyia flavipes larvae in the pasture, and the presence of Hymenoptera (sawfly) larval body fragments and heads in the contents of the forestomachs of the necropsied cattle. No hepatotoxic plants were found in the pasture. Speciation of different Parreyia spp. under field conditions is challenging, because of the lack of dichotomous keys for species identification. Identification of adult specimens is necessary to confirm the sawfly species. However, based on the regional literature, we speculate that the species involved in the outbreak was Perreyia flavipes.4
It is worth mentioning that there is evidence of chronic damage in the submitted sections of liver, including portal fibrosis, mild bile duct hyperplasia and portal hepatitis. We interpreted these as incidental pre-existing lesions, which has no direct relationship with the acute hepatotoxic condition associated with the death. Probably these chronic lesions could represent sequelae of ingestion of sublethal amounts of pyrrolizidine alkaloids, contained in some plants such as Senecio spp., or hepatic fascioliasis, which are well documented causes of chronic liver damage in the geographical area where the outbreak occurred.2,11
The consumption of larvae of insects in the order Hymenoptera ("sawfly"), should be considered as a differential diagnosis in cases of acute hepatotoxicity in cattle, sheep and pigs.2 For more than half a century cases of sawfly intoxication have been described in different parts of the world. In Australia, the intoxication is a consequence of the consumption of Lophyrotoma interrupta (family: Pergidae). The outbreaks are limited to areas with large forests of Eucalyptus melanophloia, host tree of the larva.7 In Denmark sawfly poisoning is reported in sheep due to the consumption of Arge pullata (family: Argidae), on pastures in birch forests (Betula pendula).16 In Brazil (states of Rio Grande do Sul and Santa Catarina), outbreaks had been reported in pigs, sheep and cattle, due to the consumption of larvae of Perreyia lepida (family: Pergidae), colloquially known as "mata porco".12,13,15 In Uruguay, Dutra et al. (1997) described naturally-occurring cases associated with the ingestion of Perreyia flavipes, including epidemiological aspects, clinical signs and pathology. In addition, the author reproduced the poisoning experimentally in sheep and calves and described some aspects of the biology of the adult insect.4,5
Since the discovery of the toxic effect of sawfly larvae, additional information has been generated about the biology of these insects. Adults of P. flavipes are wasps with a short life-span (<72 h), emerging in February/March.5,14 They are easily differentiated from other Perreyia species due to morphological characteristics: bright orange leg parts and 13?15 antennal articles.8 The adults do not feed. Females have little activity and fly only short distances, usually staying on the top of grasses or trying to reach an elevated position. Eggs are laid in the soil in clusters of 100?700. Eggs are oblong and yellowish-white, although once inseminated develop a blackish-yellow color.5 The incubation period is limited to 4?8 weeks, larvae start to hatch in March/April (late summer and early autumn in the Southern hemisphere), and develop through the autumn and winter.14 During the winter season, from June to September, aggregates of P. flavipes larvae are commonly found in open grasslands, forming an orderly moving column of approximately 100 individuals, particularly after rainfalls and on cloudy days. Occasionally, large numbers of these insect masses are seen moving around the ground, while other days very few or no groups are found.4
The sawfly toxin responsible for deaths of livestock had been at first reported as a hepatotoxic octopeptide containing 4 D-aminoacids, called lophyrotomine. This compound has been isolated from larvae of L. interrupta and A. pullata at concentrations of up to 0.07% of the dry matter, with a lethal dose 50 (LD50) of 2 mg/kg by intraperitoneal injection in mice.9 Subsequently, a closely related octapeptide (LGlu) and 3 heptapeptides were discovered in other sawfly species: pergidin (Perg), 4 valinepergidin (VPerg), and dephosphorylated pergidin (dpPerg).4,10 The heptapeptides contain 5 D-amino acids, and Perg and VPerg also contain phosphoserine. These compounds are highly toxic for vertebrates and have been detected and quantified in 4 sawfly species: L. interrupta, L. zonalis, P. flavipes (Pergidae), and Arge pullata (Argidae).6,10 The main toxin in P. flavipes is pergidin, a heptapeptide containing a phosphoseryl residue, although small amounts of lophyrotomin are also present.1,9 All these sawfly species can be found in large masses in the field, which facilitated the investigation and detection of the toxins. Recent studies, using a new extraction procedure that allowed testing single larvae by liquid chromatography?mass spectrometry (LC-MS) analyses, also identified the toxins in related but nonpullulating sawfly species. The study found deviations from the published species-specific chemical profiles in 3 of 4 Hymenoptera species.1 The authors concluded that intraspecific variation in the peptides analyzed, but also the methods used for chemical extractions and analyses, are possible explanations for these differing results.1
Contributing Institution:
"Plataforma de Investigación en Salud Animal, Instituto Nacional de Investigación Agropecuaria" (INIA), La Estanzuela, Uruguay.
JPC Diagnosis:
Liver: Necrosis, centrilobular and midzonal, diffuse, with hemorrhage.
JPC Comment:
The contributor provides an excellent and interesting review of sawfly larva toxicity, a condition that affects cattle and small ruminants in various geographic locations, including Australia, Denmark, and South America.2 A similar 2012 WSC case was presented in conference 15, case 1.
The first reliable report of sawfly toxicity in cattle was in the Maranoa district of Queensland, Australia in 1911, although the first suspected poisoning was reported in 1887, approximately two decades after cattle were introduced to the region. A 1982 survey of farms within the same region found the estimated annual cost to graziers to be the equivalent of $3.2 million US dollars when adjusted for inflation and current exchange rates.3
Participants discussed patterns of zonal necrosis, including centrilobular (zone 3), midzonal (zone 2), periportal (zone 1), and massive (entire lobule).2 Direct acting toxins typically cause periportal necrosis due to these hepatocytes being the first exposed. Meanwhile, centrilobular necrosis (in cases of toxicity) occurs due to the biotransformation of xenobiotics into toxic intermediates as the result of hepatocytes in this region having the highest concentration of cytochrome P450 enzymes. The moderator provided the following table of agents associated with acute hepatotoxicity in cattle:
|
Name |
Toxic Principle |
|
Blue-green algae |
Microcystin-LR |
|
Mushrooms: Amanita, Phalloides and others |
Amatoxins |
|
Cycads (Zamia sp.)Cycads (Zamia sp.) |
Methylazoxymethanol |
|
Solanacae (Cestrum sp.) |
Atractyloside |
|
Compositae (Xanthium-cockleburr) |
Carboxyatractyloside |
|
Ulmaceae (Trema sp.-Poison peach) |
Trematoxin |
|
Myoporacae (Myoporum) |
Ngaione (periportal) |
|
Iron |
|
|
Sawfly larvae (Lophyrotoma sp.) |
Lophyrotomin/pergidin |
Prior to death, clinical signs consistent with liver failure caused by a severe necrotizing hepatopathy may be observed and include acute hepatic encephalopathy, hemorrhage, jaundice, and photosensitization. Acute hepatic encephalopathy occurs due to increased plasma ammonia levels and hypoglycemia, manifesting as weakness, depression, and stupor, although some animals may become highly excited and demonstrate aggressive behavior as observed in this affected herd. Widespread hemorrhage has been reported to be variably prominent in field cases; however, studies of experimentally infected calves have found increased prothrombin and activated thromboplastin times and reduced fibrinogen concentrations. Jaundice and photosensitization are rarely observed due to the short course of the disease.4
There is bridging portal fibrosis and biliary hyperplasia within the section, as noted by the contributor. The significance of this finding was discussed amongst participants with consensus that the lesion is likely pre-existing and unrelated to sawfly larvae ingestion.
Finally, there was spirited discussion amongst participants in regard to use of the word "diffuse" in the morphologic diagnosis due to risk of confusion with massive necrosis, which was not seen in this case. Massive necrosis refers to necrosis of an entire hepatic lobule, not necessary the liver as a whole.2 Therefore, when describing zonal necrosis, one should strive to avoid such confusion by including a zonal distribution modifier in addition to the overall distribution within the examined section.
References:
1. Boevé JL, Rozenberg R, Shinohara A, Schmidt S. Toxic Peptides Occur Frequently in Pergid and Argid Sawfly Larvae. PLOS ONE. 2014. 9(8): e105301. https://doi.org/10.1371/journal.pone.0105301
2. Cullen JM, Stalker MJ. Liver and biliary system. In: Maxie MG. ed. Jubb, Kennedy and Palmer 's Pathology of Domestic Animals. 6th ed. Vol 2. Saunders Elsevier. 2016:284, 330-332.
3. Dadswell LP, Abbott WD, McKenzie RA. The occurrence, cost and control of sawfly larval (Lophyrotoma interrupta) poisoning of cattle in Queensland 1972-81. Aust Vet J. 1985;62(3):94-97.
4. Dutra F, Riet-Correa F, Mendez MC, Paiva N. Poisoning of cattle and sheep in Uruguay by sawfly (Perreyia flavipes) larvae. Vet Hum Toxicol. 1997;39(5):281-286.
5. Dutra F. Intoxicación por larvas de Perreyia flavipes en bovinos y ovinos. Caracterización de la enfermedad y biología del insecto. Veterinaria (Montevideo). 2003;38 (152-153):7-24. In Spanish.
6. MacLeod JK, Braybrook C, Simmonds PM, Oelrichs PB. A unique toxic peptide from the larvae of the South American sawfly, Perreyia flavipes. Aust J Chem. 2000;53:293?297.
7. McKenzie RA, Dunster PJ, Twist JO, Dimmlock CK, Oelrichs PB, Rogers RJ, Reichmann, KG. The toxicity of sawfly larvae (Lophyrotoma interrupta) to cattle. Qld. Dept. Prim. Ind. Bull. (1985). QB85001.
8. Neves, F.M., Pie, M.R. On the Adult Behavioral Repertoire of the Sawfly Perreyia flavipes Konow, 1899 (Hymenoptera: Pergidae): Movement, Mating, and Thanatosis. Neotrop Entomol. 2018;47:46?52.
9. Oelrichs PB, MacLeod JK, Seawright AA, et al. Unique toxic peptides isolated from sawfly larvae in three continents. Toxicon. 1999;37(3):537-544.
10. Oelrichs PB, MacLeod JK, Seawright AA, Grace PB. Isolation and identification of the toxic peptides from Lophyrotoma zonalis (Pergidae) sawfly larvae. Toxicon. 2001;39(12):1933-1936.
11. Preliasco M, Rivero R. Poisoning of Cattle by Senecio spp. in Uruguay. In: Poisoning by plants, mycotoxins, and related toxins. 2009;30:198-207.
12. Raymundo DL, Bezerra Jr. PS, Bandarra PM, Pedroso PMO, de Oliveira EC, Pescador CA, Driemeier D. Spontaneous poisoning by larvae of Perreyia flavipes (Pergidae) in sheep. Pesq. Vet. Bras. 2008. 28 (1): 19-22.
13. Raymundo DL, Bezerra Jr. PS, Bandarra PM, Dalto AGD, Soares MP, da Cruz CEF, Driemeier D. Perreyia flavipes larvae toxicity. Pesq. Vet. Bras. 2012; 32(8):735-738.
14. Soares MP, Riet-Correa F, Smith D, Pereira Soares M, Mendez MC, Brandolt AL. Experimental intoxication by larvae of Perreyia flavipes Konow, 1899 (Hymenoptera: Pergidae) in pigs and some aspects on its biology. Toxicon. 2001; 39:669?678.
15. Soares MP, Quevedo PS, Schild AL. Intoxicacao por larvas de Perreyia flavipes em bovinos na regiao sul do Rio Grande do Sul. Pesq. Vet. Bras. 2008;28(3):169-173. In Portuguese.
16. Thamsborg SM, Jørgensen RJ, Brummerstedt E. Sawfly poisoning in sheep and goats. Vet Rec. 1987;121(11):253-255.