WSC 2023-2024, Conference 22, Case 4
Signalment:
8-year-old female intact American Staffordshire terrier (Canis familiaris).
History:
The dog presented to the small animal hospital with acute onset of tachypnea, abdominal breathing, tachycardia, and bright red mucous membranes. The dog had been coughing for a month, had been inappetent, exercise intolerant, and had been losing weight for 1-2 weeks. Two weeks prior, the dog was diagnosed with a urinary tract infection. The dog was euthanized without further diagnostics and subsequently submitted for necropsy.
Gross Pathology:
The dog was in good body condition. Originating from the mucosa of the trigone area of the urinary bladder was an irregularly and indistinctly outlined mass, approximately 6 cm in diameter, with homogenous white to yellow colour, firm consistency, and a smooth, solid white cut surface. The mass extended into and infiltrated nearly the full length of the urethra. The inguinal lymph nodes were severely enlarged with a homogenous light colour, firm consistency, and a smooth, solid cut surface. Bilaterally in the adrenal medulla, 0.5 cm diameter, well-demarcated masses of similar appearance were found. Multifocally within all lobes of the lungs were abundant nodular, well demarcated masses of light colour, firm consistency, and smooth, solid cut surface, ranging from 0.3-2.0 cm in diameter. The tracheobronchial and mediastinal lymph nodes displayed a similar appearance as the inguinal lymph nodes.
Microscopic Description:
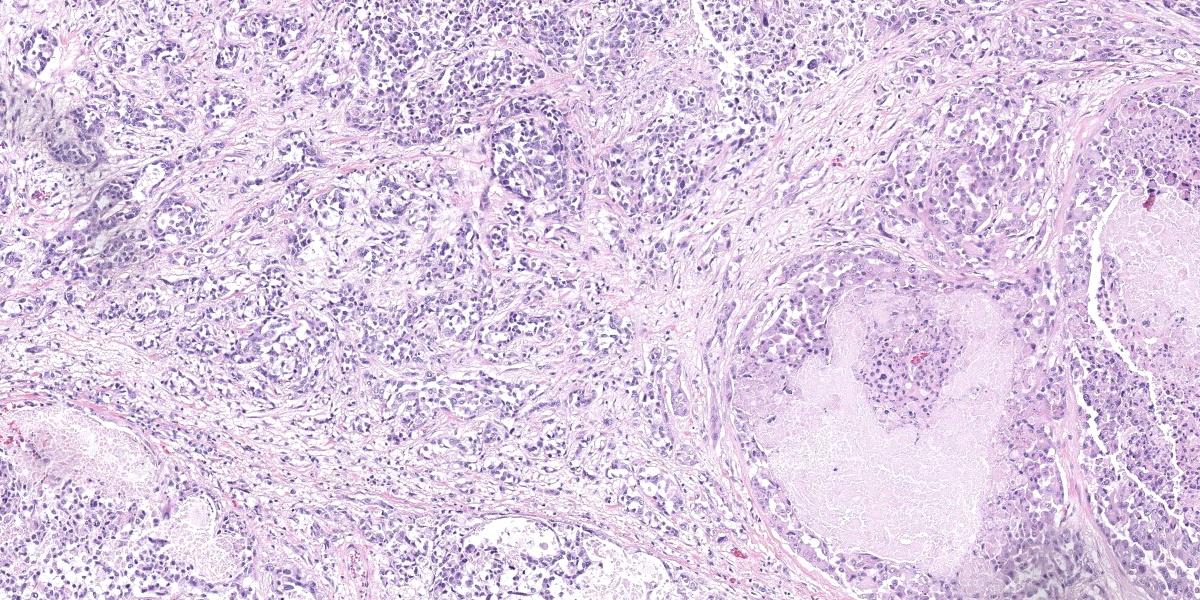
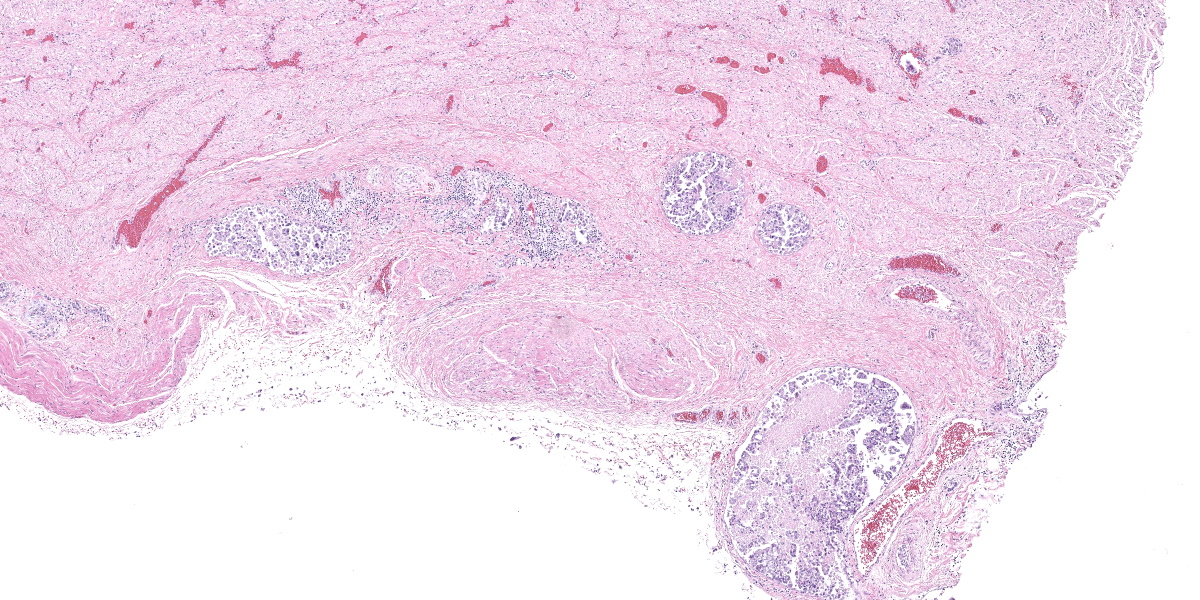
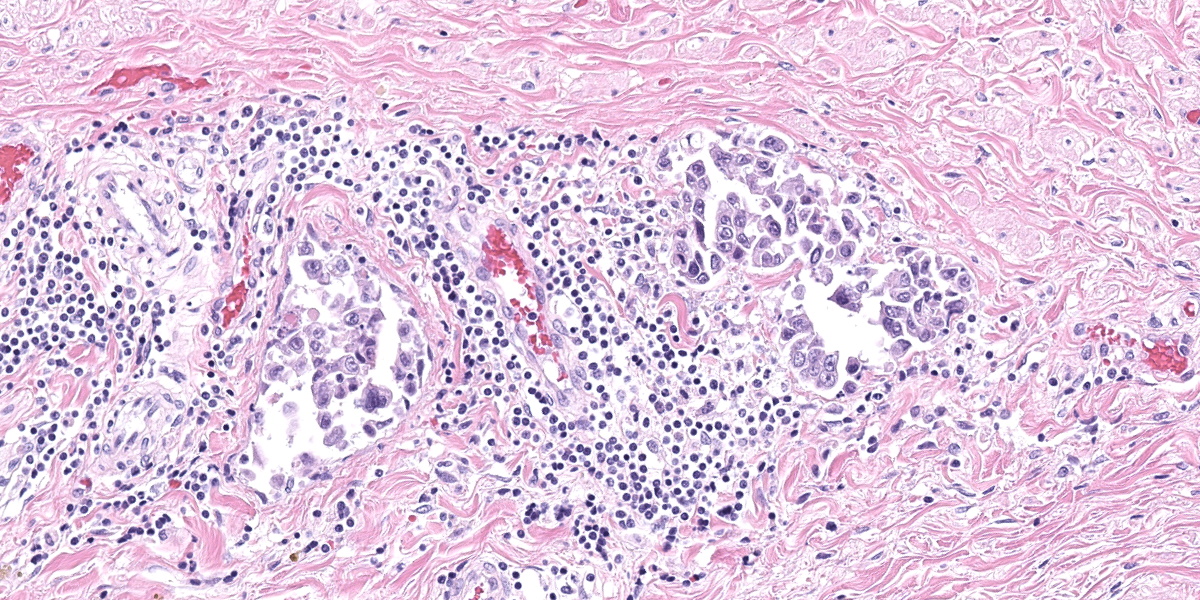
Urinary bladder: Diffusely obliterating and irregularly thickening the mucosa and extending into the submucosa is a plaque-like, poorly demarcated and unencapsulated, infiltrative, densely cellular epithelial neoplasm, extending to cut borders. A few scattered neoplastic cells are also seen within the muscular layer. Neoplastic cells are polygonal, markedly pleomorphic, and grow in cords, tubules, and poorly defined aggregates in a scant to moderate fibrovascular stroma. Nuclei are large, round to oval with vesicular chromatin and 1-2 central, prominent nucleoli. Multiple multinucleated cells and cells with bizarre, giant nuclei are seen. The cytoplasm is moderate, eosinophilic, and homogenous to vacuolated, and cell borders are mostly distinct. The N:C ratio is increased. Multifocally, neoplastic cells exhibit large intracytoplasmic vacuoles with eccentric peripherally located nuclei (signet ring formation) or large intracytoplasmic vacuoles containing homogenous or granular eosinophilic, PAS-positive material (Melamed-Wolinska bodies). The mitotic activity is high (25 mitoses per 10 HPF) and atypical mitoses are present. Multifocally, neoplastic cells display pyknotic or karryorhectic nuclei (single cell necrosis) and there are multifocal larger, confluent areas of necrosis. In the muscularis layer are multiple tumoral emboli within thin-walled vessels (lymphovascular invasion).
Contributor’s Morphologic Diagnosis:
Urinary bladder: Urothelial carcinoma, non-papillary and infiltrative, high grade.
Contributor’s Comment:
Tumors of the urinary bladder are uncommon in dogs, accounting for ≤1% of all canine neoplasms and 2% of all malignant canine neoplasms.3,7 Lower urinary tract carcinomas have four histological types: (1) urothelial, (2) squamous, (3) adenocarcinoma, or (4) undifferentiated carcinoma.3 Of these, the urothelial carcinoma (UC), previously called transitional cell carcinoma (TCC), is the most common bladder neoplasm in all species including dogs.7 UC is derived from the transitional epithelium of the urinary tract (urothelium).7 UC also occurs in cats and cattle, and may develop in cattle as a sequela to ingestion of bracken fern (enzootic hematuria).3 Bladder neoplasms of any type are rare in other species.7
Urothelial carcinoma occurs primarily in older dogs (average age 9-11 years), and females seem to be more often affected than males, however a statistically significant gender difference is lacking.7 Neutered dogs seem to be predisposed to bladder neoplasms.7 Scottish terriers have an 18 to 20-fold higher risk for UC than other dogs. Other breeds at higher risk are Airedale terriers, Shetland sheepdogs, West Highland white terriers, fox terriers and beagles.7
Most of the UC neoplasms in dogs occur in the trigone area of the bladder, possibly because of a prolonged contact time of urine and potential carcinogens with the urothelium in this area.7 In 30-55% of bladder UCs there will be concurrent neoplastic lesions in the prostate and/or urethra.7 Grossly, most tumors are solitary, but they can be multiple and cover nearly the entire urinary bladder mucosa.7 They may form either papillary growths projecting into the lumen of the bladder or non-papillary, flat plaques or masses that bulge from the mucosa into the bladder lumen.7 Most are infiltrative into the muscle layers, producing a thickened bladder wall.7
A histological grading scheme is available for histological diagnosis of canine UC. The grading scheme has been based largely on the World Health Organization (WHO) histological criteria for human UCs, and was last modified in 2012.1,7 In the classification scheme for dogs, UC is divided into low and high-grade variants.1,7 The most aggressive region of the tumor should be used to assign a grade.7 High-grade UCs are defined by features of malignancy such as disorganized growth and loss of cell polarity, cellular atypia, nuclear pleomorphism, mitotic activity, deep invasion and invasion into lymphatics or blood vessels.1,7 Low-grade UCs display no invasion and are confined to the mucosa, exhibit mild pleomorphism, and contain no or only a few mitoses.7 The great majority of UC/TCC are high-grade and invasion is present in the majority of canine UCs (>90%).7 However, studies assessing the prognostic relevance of the grading scheme are lacking, and some studies have shown no correlation between histologic variables and prognosis.1,11 Prospective studies determining the relationship between low- and high-grade features are needed to know if the grading scheme for UC accurately predicts clinical outcomes in dogs.1,7
Canine UCs can also be divided based on morphological pattern of growth, namely papillary (projecting into the lumen, around 50% of cases) or non-papillary (sessile or flat, 50%), and infiltrating (90%) or non-infiltrating (10%). This designation should be based on the dominant gross and microscopic features.7 In a case series of 3 canine UCs, unique additional cellular morphologies were revealed, resembling human plasmacytoid and rhabdoid variants of UC.5 Reduced E-cadherin expression was shown by immunohistochemistry (IHC) in 2 of the cases, possibly representing increased invasiveness and epithelial-mesenchymal transition (EMT).5
Keys to the histological diagnosis of UC are location in the urinary bladder, presence of large epithelial cells with multiple cellular and nuclear features of atypia, invasion below the mucosa or into lymphatics or blood vessels, and the presence of so-called Melamed-Wolinska bodies.7 Melamed-Wolinska bodies are large cytoplasmic vacuoles that may be empty or contain homogenous or stippled periodic acid Schiff (PAS) positive eosinophilic material, as was confirmed in this case. The Melamed-Wolinska bodies are also uroplakin positive and are so characteristic of UC that if seen in preparations from other locations such as lymph node, skin, or abdominal or pleural fluid, UC should be listed as the most likely differential diagnosis.7 Urothelial tumor cells typically exhibit eosinophilic cytoplasm, large and vesicular nuclei, and numerous mitoses, some of which are bizarre. Multinucleated cells are common. Regions of squamous and/or glandular metaplasia and signet ring cell formation may occur, as well as desmoplasmic reactions.7
High-grade, invasive UCs are one of the most aggressive neoplasms in veterinary medicine and the prognosis is poor.4,7 Survival times are short for almost all UCs in dogs, and most dogs (>80%) die within the first year of treatment.4,7 Metastases occur most commonly to the lungs and regional lymph nodes, but may also occur to bones and skin.7 Additional metastases may be found in almost any organ examined.7 In a retrospective study, 17 of 188 canine UC cases (9%) had histologically confirmed skeletal metastasis, mainly affecting vertebrae.2 Possible routes of metastases of canine UCs include vascular dissemination with tumor emboli, direct extension (peritoneal implantation) from transmural bladder infiltration, or iatrogenic seeding through diagnostic sampling by fine needle aspirate or surgery.7
Implantation of tumor along surgical excision paths is well documented with UC.7 In one study, skin metastases were seen near the vulva or prepuce, possibly because of urine scalding of the skin and subsequent transepidermal spread.10 Clinically, bladder carcinomas in dogs are staged with the Tumor Nodes Metastasis (TNM) system.7 At the time of clinical diagnosis, 20% of dogs will have radiographically detectable metastases, and metastases are present in a majority of dogs (50-90%) at autopsy.4,7
Special genetic profiles seem to be associated with the development of canine UC.7 Studies of canine UC biopsies have revealed aberrant chromosomal numbers (aneuploidy) of Canis familiaris (CFA) chromosome 13, 19, and 36, with gain of CFA13 and 36 and loss of CFA19.13 Aberrant copy number variations in the same chromosomes were detected also by droplet digital polymerase chain reaction (ddPCR).8 In addition, a digital PCR assay has been used to detect copy number aberrations (CNAs) in the ErbB2 (HER2) oncogene, whose product, the cell surface receptor tyrosine kinase ErbB2, is overexpressed in a variety of human malignancies.12 In the study, ErbB2 copy number aberrations were detected in 33% of UC bladder tissue, and in 35% of UC urinary sediment, meanwhile no ErbB2 CNAs were detected in normal controls.12 In a immunohistochemical study performed on 23 samples of canine UCs, intense membranous ErbB2 (HER2) immunoreactivity was frequently observed in neoplastic cells.16 In another study, 36 of 47 dogs (76.6% with UCs) had a mutation in proto-oncogene B-raf (BRAFV595E mutation).6 The mutation was associated with tumor-produced chemokine CCL17 and infiltration regulatory T-cells, which may act as immune suppressors but also are thought to play a role in tumor progression.6
Immunohistochemistry is rarely needed to diagnose primary UC unless the tumor is anaplastic.7 If needed, uroplakin III (UPIII) is considered the best IHC marker to recognize urothelium, and is a useful primary biomarker to identify tumors of suspected urothelial origin.7 Uroplakin consists of four proteins located in a specialized plasma membrane (asymmetrical unit membrane, AUM) that connects the surface of urothelial cells with cytoplasmic filaments.7 UPIII is specific to terminally differentiated superficial urothelial cells (umbrella cells) and exhibits discrete membranous immunoreactivity, primarily at the surface of cell membranes.7 UPCIII cannot be used to differentiate neoplastic from non-neoplastic lesions, but it is highly specific for urothelial tumors, and may be helpful to rule in or out UC origin in metastases or cancer of unknown primary site.7 Due to its high specificity in canine urothelial neoplasm, UPIII expression in less than 5% of neoplastic cells is considered diagnostic.7 UPIII labeling may however be lost in some high-grade, anaplastic UCs, possibly due to loss of cell adhesion molecules, enhancing the ability to metastasize.7 Lack of immunoreactivity may therefore indicate a more aggressive tumor.7
Antibodies to uroplakin II have been evaluated in human pathology and have been shown to outperform UPIII.15 Urothelium (transitional epithelial cells) also contain cytokeratins associated with simple-type epithelium (CK7, CK20, CK8, CK18, CK19) and stratified-type epithelium (CK13, CK17). CK7 and CK20 have been used in UC diagnosis in humans.7 In a canine study, CK7 and UPIII outperformed CK20 in the diagnosis of UC.9 CK7 staining was diffusely cytoplasmic and stained over 98% of primary UCs, whereas UPIII labeled 91% of primary UCs.9 Another potential immunohistochemical marker for canine UC is cyclooxygenase-2 (COX-2). COX-2 and associated production of prostaglandin E2 have been ascribed several roles in carcinogenesis, including immunosuppression, increased metastatic potential of neoplastic epithelial cells, and stimulation of angiogenesis.14 Immunoreactivity for COX-2 has been detected in UCs of dogs, but also in non-neoplastic proliferative bladder lesions.14
Contributing Institution:
Swedish University of Agricultural Sciences
Department of Biomedical Sciences and
Veterinary Public Health
Pathology Section
Uppsala, Sweden
https://www.slu.se/en/departments/biomedical-sciences-veterinary-public-health/
JPC Diagnosis:
Urinary bladder: Urothelial carcinoma, papillary and infiltrative.
JPC Comment:
The contributor provides an excellent overview of the extensively researched and described canine urothelial carcinoma. Interestingly, despite UC also being the most common bladder tumor in cats, far less has been published about feline UC. Feline UC comprises only about 0.5% of all feline malignancies, compared with around 2% of all canine malignancies, and this difference has been speculatively explained by, among other variables, the lower quantities of tryptophan metabolites excreted in feline urine, differences in the chemical composition of feline flea and tick preventatives, and potential masking by concurrent disease in aged cats.17
A recent descriptive study of feline UC in 38 cats found that the typical histologic presentation is of an unencapsulated, densely cellular neoplastic mass, often with ulceration or erosion of the bladder mucosa.17 As in canine UC, the two main histologic patterns reported are papillary, exophytic growths extending into the bladder lumen (21%) or the formation of non-papillary sessile growths (79%).17 The case series found evidence of Melamed-Wolinska bodies or signet ring formation in only 3 of 38 cases, perhaps indicating that this histologic finding is less frequent in feline cases compared with canine UC. Mitotic rate was variable with occasional bizarre mitotic figures.17
Neoplastic cells are typically arranged in similar patterns, such as trabeculae, cords, and islands, as their canine UC counterparts; however, feline UCs frequently (9/38) contain a glandular histomorphology that appears similar to a histologic variant seen in humans, but not dogs, and researchers suggest that this unique morphology should be described in pathologic reports of feline UC to aid in identifying metastatic disease that may show this unexpected pattern.17 Feline UC neoplastic cells are present on variable stroma that ranges in quantity and morphology; stroma may be sparse or abundant, and may be characterized as mucinous, myxomatous, or scirrhous. Inflammatory infiltrates, when present, are primarily composed of lymphocytes with fewer plasma cells and neutrophils.17
There are some reported associations between feline UC and urinary tract infection, with concurrent infections reported in up to 70% of feline UC patients.7,17 In the most recent case series, approximately 28% of patients for whom clinical history was available had cystitis, stones or bladder crystals at the time of diagnosis.17
Though data is sparse, feline UC is thought to be highly recurrent, and metastasis at the time of diagnosis, typically to the regional lymph nodes or lungs, has been reported in 12-50% of cases. As the contributor notes, the prognosis for canine UC is strongly associated with the TNM staging system; however, TNM staging is typically not performed with feline UCs and prognostic information has therefore not been established.17
As in the previous case, Dr. Meuten chose to discuss this classic neoplasm in the context of synoptic reporting. Participants discussed whether infiltration, grade, or both should be reported. Dr. Meuten noted, as does the contributor, that there is no veterinary research that ascribes prognostic or clinical meaning to UC grading, and he would therefore be inclined to omit this data. This lead to a broader discussion of how synoptic reporting could potentially be tailored to meet the needs of the intended recipient, as information considered useful by a general practitioner is likely different than what would be useful for a veterinary oncologist. Conversation broadened to discuss which stakeholders are best positioned to generate synoptic reporting templates. Should it be the pathologists, who are not necessarily up to date on the latest prognostic, therapy-determinative factors for all tumors in veterinary oncology, or should it be the oncologist, who are more knowledgeable on those points and who are, after all, the end users. Dr. Meuten felt strongly that collaborative effort was needed between veterinary oncologists and pathologists to ensure that any synoptic report templates are targeted, useful, and relevant to the widest audience possible.
As discussed above, the authors of the most recent descriptive feline UC study noted that a particular histomorphologic feature (glandular morphology) should be reported in veterinary reports, not because it had prognostic value, but because it had potential utility in identifying metastatic disease with an unexpected histomorphology. This data point, not prognostic and not backed up by extensive research yet nonetheless useful, gets at the heart of many conference participants’ concerns over the trend toward synoptic reporting. A checklist, “just the facts” style of report, while perhaps efficient, easy to read, streamlined, and standardized, feels as if it strips the profession of something fundamental: the ability to communicate impressions and findings, born not solely from metrics, but from experience, that others may find useful when practicing the art of medicine. As synoptic reporting continues to gain popularity, discussions such as these should continue to ensure that nuanced evaluation and communication are not sacrified on in the interest of data-driven efficiency.
On a more prosaic note, conference participants preferred to omit grading from the diagnosis as, per conference discussion, the histologic grade currently has no prognostic significance. Conference participants noted the contributor’s classification of the UC as non-papillary; however, in the section examined at conference, participants felt that the dominant pattern was papillary, and this view is reflected in the morphologic diagnosis.
References:
- Avallone G, Rasotto R, Chambers JK, et al. Review of histological grading systems in veterinary medicine. Vet Pathol. 2021;58(5):809-828.
- Charney VA, Miller MA, Heng HG, Weng HY, Knapp DW. Skeletal metastasis of canine urothelial carcinoma: pathologic and computed tomographic features. Vet Pathol. 2017;54(3):380-386.
- Cianciolo RE, Mohr FC. Urinary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domesticated Animals. 6th ed. Elsevier;2016:461-463.
- Cullen JM, Breen, M. An overview of molecular cancer pathogenesis, prognosis and diagnosis. In: Meuten DJ, ed. Tumors in Domestic Animals. Wiley-Blackwell;2017 :1-26.
- Je-Han Lin, Kao CF, Wang FI, et al. Urothelial carcinomas of the urinary bladder with plasmacytoid or rhabdoid features and tendency of epithelial-mesenchymal transition in 3 dogs. Vet Pathol. 2018;55(5):673-677.
- Maeda S, Yoshitake R, Chambers JK, et al. BRAFV595E mutation associates CCL17 expression and regulatory T cell recruitment in urothelial carcinoma of dogs. Vet Pathol. 2021;58(5):971-980.
- Meuten DJ, Meuten TLK. Tumors of the urinary system. In: Meuten DJ, ed. Tumors in Domestic Animals. Wiley-Blackwell; 2017:632-688.
- Mochizuki H, Shapiro SG, Breen M. Detection of copy number imbalance in canine urothelial carcinoma with droplet digital polymerase chain reaction. Vet Pathol. 2016;53(4):764-772.
- Ramos-Vara JA, Miller MA, Boucher M, Roudabush A, Johnson GC. Immunohistochemical detection of uroplakin III, cytokeratin 7 and cytokeratin 20 in canine urothelial tumors. Vet Pathol. 2003;40:55-62.
- Reed LT, Knapp DW, Miller MA. Cutaneous metastasis of transitional cell carcinoma in 12 dogs. Vet Pathol. 2012;50(4): 676-681.
- Rocha TA, Mauldin GN, Patnaik AK, Bergman PJ. Prognostic factors in dogs with urinary bladder carcinoma. J Vet Intern Med. 2000;14:486-490.
- Sakai K, Maeda S, Saeki K, et al. ErbB2 copy number aberration in canine urothelial carcinoma detected by a digital polymerase chain reaction assay. Vet Pathol. 2020;57(1):56-65.
- Shapiro SG, Raghunath S, Williams C, et al. Canine urothelial carcinoma: genomically aberrant and comparatively relevant. Chromosome Res. 2015; 23:311-331.
- Sledge DG, Patrick DJ, Fitzgerald SD, Xie Y, Kiupel M. Differences in expression of uroplakin III, cytokeratin 7 and cyclooxygenase-2 in canine proliferative urothelial lesions of the urinary bladder. Vet Pathol. 2015;52(1):74-82.
- Smith SC, Mohanty SK, Kunju LP, et al. Uroplakin II outperforms uroplakin III in diagnostically challenging settings. Histopathology. 2014;65:132-138.
- Tsuboi M, Sakai K, Maeda S, et al. Assessment of HER2 expression in canine urothelial carcinoma of the urinary bladder. Vet Pathol. 2019;56(3):369-376.
- van der Weyden L, O’Donnell M, Plog S. Histological characterization of feline bladder urothelial carcinoma. J Comp Pathol. 2021;182:9-14.