Signalment:
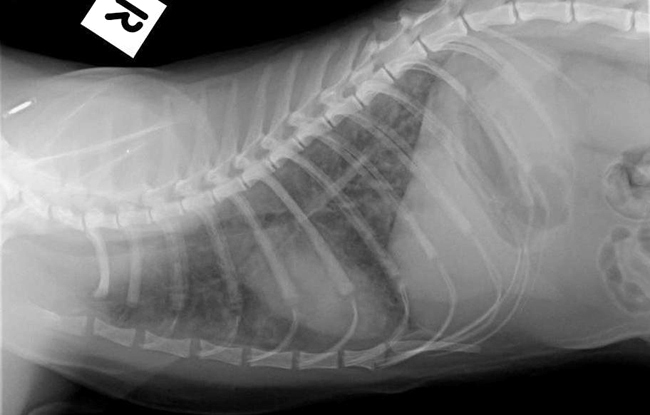
Gross Description:
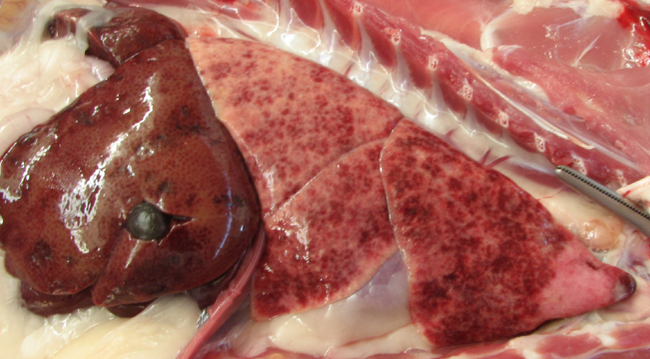
The liver had a pronounced acinar pattern. Multifocal irregularly shaped maroon discolorations, which were often depressed, were present on the capsular surface (Fig 3).Â
All other organ systems were examined with no further gross lesions detected.Â
Histopathologic Description:
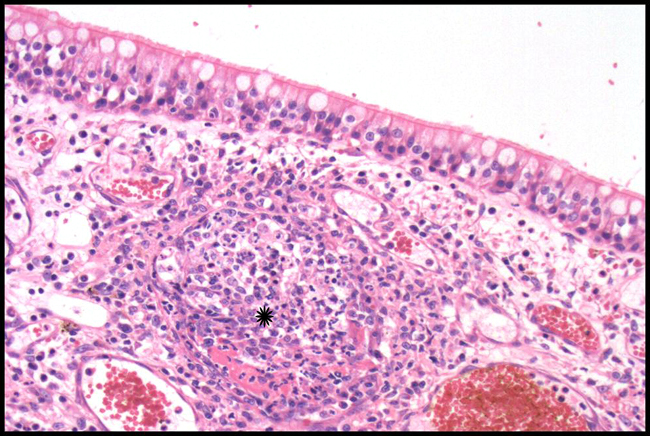
Lung: there was a diffuse interstitial pneumomia with widely distributed foci of intense pyogranulomatous inflammation. Multifocal moderately sized areas of parenchyma were necrotic and surrounded by fibrinous effusion.Â
Liver: there was severe diffuse hydropic change of hepatocytes with mild bridging fibrosis and mild periportal lymphoplasmacytic inflammatory infiltrates. Multifocal moderately sized areas of telangiectasia were present and most noticeable towards the capsular surface. Leukocyte numbers were increased within the sinusoids.Â
Lymph nodes: Cortices contain numerous follicles with prominent germinal centres. The paracortices were expanded by numerous small mature lymphocytes. Numerous plasma cells were present within the medullary cords. There was a sinus histiocytosis. Multifocal granulomas of varying size were scattered throughout the nodes.Â
Bone marrow: there was a marked increase in myeloid to erythroid ratio
Morphologic Diagnosis:
Lymph nodes: Lymphadenitis, granulomatous, chronic, multifocal, moderately severe.
Lab Results:
Bronchoalveolar lavage contained large numbers of degenerate nucleated cells and an abundance of cellular debris. Markedly increased numbers of neutrophils (85%) and an abundance of mucous were also noted.Â
Serology
| Toxoplasmosis IFAT (IgG) | negative |
| Toxoplasmosis IFAT (IgM) | negative |
| Feline Calici Virus IFA Titre | >= 1:80 |
| Feline Herpes Virus IFA Titre | = 1:20 |
| Feline Panleukopaenia Virus IFA Titre | = 1:20 |
| Feline Coronavirus IFA Titre | >= 1:2560 |
| Feline Immunodeficiency Virus PCR | negative |
Condition:
Contributor Comment:
Feline infectious peritonitis (FIP) is a worldwide fatal systemic disease of wild and domestic felids which is triggered by infection with FCoV. The disease syndrome was first described in 1963 with the aetiological agent identified in 19783 . Granulomatous vasculitis and perivasculitis (especially of venules), fibrinous to granulomatous serositis, pyogranulomatous inflammation and granulomas in organs are characteristic histopathological lesions.1,3,4 These result in the spectrum of wet (with large amounts of highly proteinaceous cavitory effusions) to dry (multifocal granulomas) clinical presentations.Â
FCoV is a member of the family Coronaviridae, genus Coronavirus. Coronaviruses are enveloped positive stranded RNA viruses averaging 100 nm in diameter and have characteristic petal shaped surface projections (peplomers) which are responsible for the crown like (corona) electron microscopic appearance.3 Corona viruses have relatively low species specificity. Canine coronavirus is closely related to FCoV and can infect cats causing diarrhoea. Exposed cats develop antibodies which cross-react with FCoV. Infection with FCoV results in a spectrum of outcomes, from asymptomatic enteric infection and healthy lifelong carrier status, through to systemic enteric infection to virulent systemic infection (FIP).Â
Coronavirus antibodies are present in up to 90% of cats in catteries and in up to 50% of cats in single cat households.3 However, FIP morbidity is low, with only approx 5% of FCoV infected cats developing FIP 3,4, and those cats that do develop the disease are usually under 2 years of age.1 Infection usually takes place oral-nasally from exposure to FCoV containing faeces. Transmission in saliva can occur as the virus replicates in the tonsils in the early stages of infection.3 Respiratory droplet and urine are also considered possible vehicles for transmission.3 Transplacental transmission has been shown to occur but is rare.3 FCoV replicates in enterocytes either asymptomatically or causing transient and usually clinically mild diarrhoea and/or vomiting.3 A short episode of mild upper respiratory tract disease may also occur.3
FIP only ensues in individual affected animals when FCoV undergoes spontaneous mutation during replication. Deletions in open reading frames 3 and 7 which code for non structural proteins are responsible for surface changes which allow the virus phagocytosed by macrophages to bind to the ribosomes of the macrophages.3,4 Mutated viruses have a 99.5% genetic homology with the parent virus.3 The mutation allows the virus to replicate within macrophages. FCoV infected monocytes are considered responsible for viral dissemination within the host.4 FCoV viraemia is detectable in both virulent (mutated FIP infection) and non mutated FCoV infected cats.3,4
The pathogenesis of FIP is complex. The virus itself does not cause major cytopathic damage; rather lesions result from the hosts own immune response.3 Fibrinogen, C3 and viral antigen demonstrated within FIP lesions, and evidence of FCoV-specific immune complexes within the blood and vessels, together with high levels of gamma globulin in affected cats support an immune complex-mediated, type III hypersensitivity pathogenesis.3,4 However, an alternative pathogenesis has been proposed where lesion initiation appears to begin with endothelial adherence and extravasation of FCoV positive, activated monocytes and progresses to venous and perivenous, macrophage dominated, focal to circular infiltrates.4 The presence of a peripheral rim of B lymphocytes in older FIP lesions, the paucity of neutrophils and T lymphocytes within the lesions and a lack of non-specific perivascular lymphocytic cuffing during one study are cited as significant differences between FIP and classical immune-complex mediated vasculitides.4
A definitive antemortem diagnosis of FIP is often challenging, complicated by the often insidious onset and non-specific signs displayed by infected animals. Laboratory tests including FCoV antibody titre (blood and CSF), RT-PCR to detect virus, ELISA to detect antibody-antigen complexes and measurement of acute phase proteins (mainly serum alpha-1 acid glycoprotein) are relatively insensitive and/or poorly specific.3,4 This is mainly due to the inability to differentiate seroconversion or viraemia caused by FCoV from that caused by mutated FCoV (FIP causing) strains. Serum alpha-1 acid glycoprotein has been shown to be elevated in other inflammatory conditions. Histopathology with immmunohistochemistry to detect FCoV infected macrophages remains the only conclusive means of diagnosing FIP.1,4
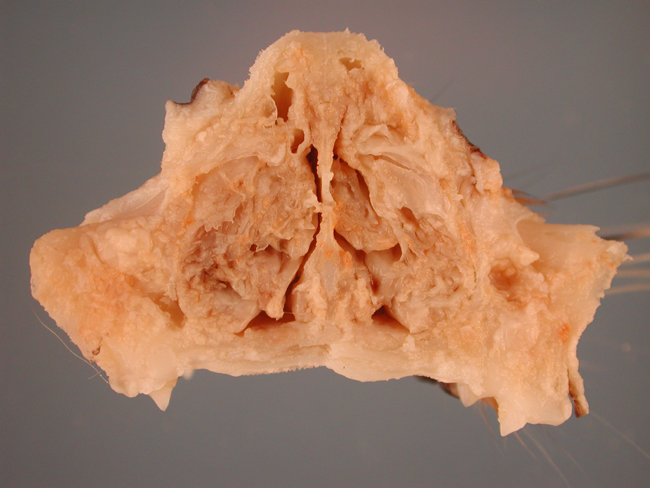
This case demonstrates classic characteristic FIP vasocentric granulomatous lesions. It is unusual in that the most severe lesions are within the nasal submucosa, this distribution has not previously been reported.Â
JPC Diagnosis:
Conference Comment:
The diseases that cause vasculitis in animals are summarized in a table below from Pathologic Basis of Veterinary Disease.5
Causes of Vasculitis in Animals
VIRAL
Equine viral arteritis (arterivirus), malignant catarrhal fever (gammaherpesvirus), hog cholera (porcine pestivirus), feline infectious peritonitis (coronavirus), bluetongue (orbivirus), African swine fever (asfarvirus), equine infectious anemia (lentivirus), bovine virus diarrhea (bovine pestivirus)
BACTERIAL
Salmonellosis, erysipelas (Erysipelothrix rhusiopathiae), Hemophilus spp. infections (Hemophilus suis, Histophilus somni, Hemophilus parasuis)
MYCOTIC
Phycomycosis, Aspergillosis
PARASITIC
Equine strongylosis (Strongylus vulgaris), dirofilariasis (Dirofilaria immitis), spirocercosis (Spirocerca lupi), onchocerciasis, elaeophoriasis (Elaeophora schneideri), filariasis in primates, aelurostrongylosis, angiostrongylosis
IMMUNE-MEDIATED
Canine systemic lupus erythematosus, rheumatoid arthritis, Aleutian mink disease (parvovirus), polyarteritis nodosa, lymphocytic choriomeningitis, drug-induced hypersensitivity
References:
2. Gelberb HB: Alimentary system. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., p. 380-381. Mosby Elsevier, St. Louis, Missouri, 2007
3. Hartmann K: Feline infectious peritonitis. Vet Clin Small Anim 35:39-79, 2005
4. Kipar A, May H, Menger S, Webber M, Leukert W, Reinacher M: Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Vet Pathol 42:321-330, 2005
5. Van Vleet JF, Ferrans VJ: Cardiovascular system. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., p. 606. Mosby Elsevier, St. Louis, Missouri, 2007
6. Zachary JF: Nervous system. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., p. 883-884. Mosby Elsevier, St. Louis, Missouri, 2007