CASE III: 5239 (JPC 4135744)
Signalment:
Adult, female, Guineafowl puffer (Arothron meleagris)
History:
This is a wild-caught, adult, female, guineafowl puffer that arrived into quarantine 6 months prior to presentation. The animal was placed into a 100,000 gallon mixed indopacific teleost and elasmobranch exhibit after a 30-day period of quarantine. After 4 months on exhibit keepers reported decreased appetite, lethargy, self-isolation and possible intraspecific aggression toward the fish. The animal was transferred to a hospital tank for examination and treatment.
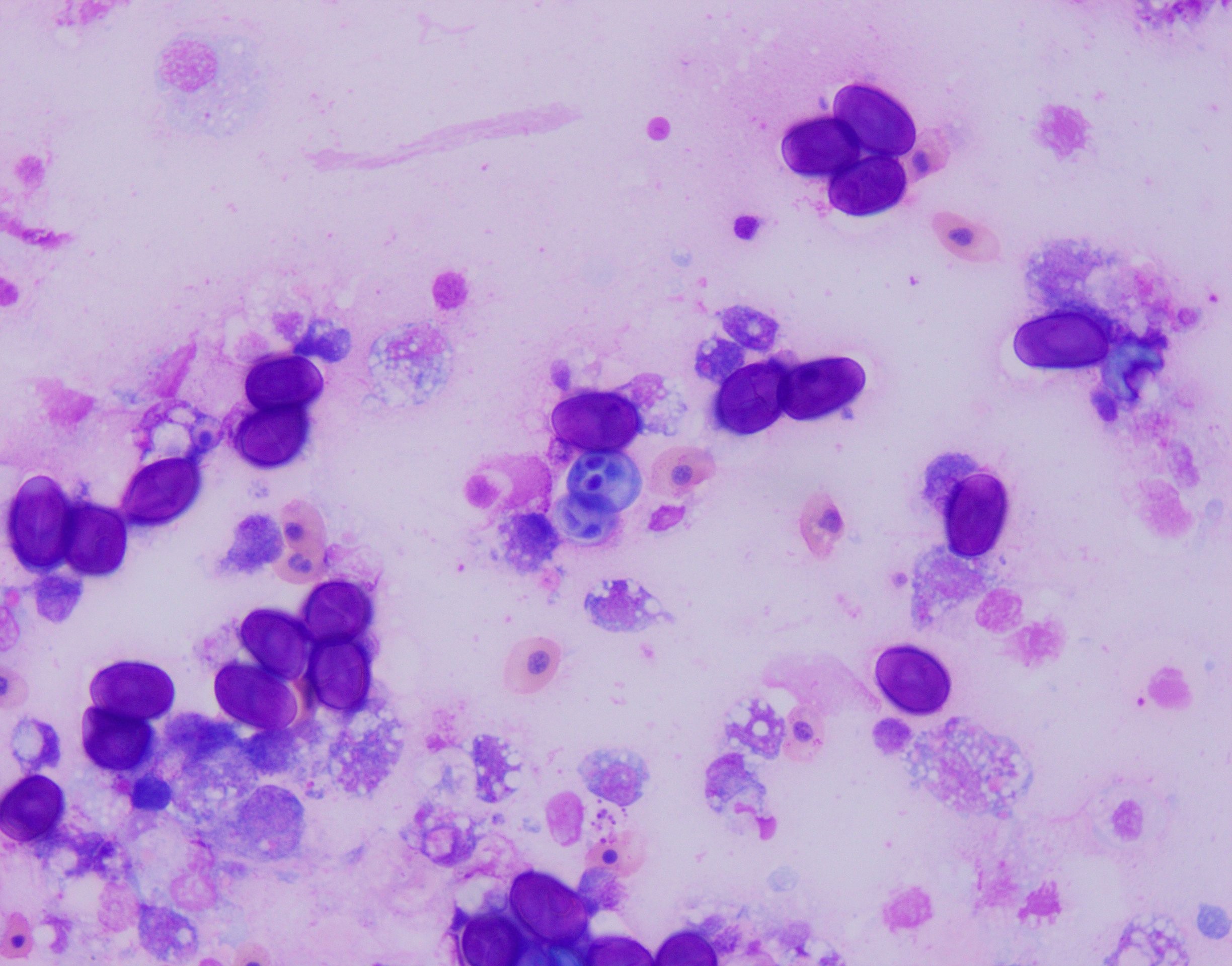
Examination revealed bilateral nodular, tan masses in the anterior chamber of the eyes, near the junction between the iris and sclera. Fine needle aspiration recovered numerous oval to round darkly staining, refractile structures intermixed with histiocytic and granulocytic inflammatory cells (Fig.1). Acid fast staining revealed these structures were strongly acid fast positive with prominent bilateral polar capsules consistent with intraocular myxozoan infection. Additionally, the animal was negatively buoyant at exam and radiographs revealed a small asymmetrical/irregular swim bladder suggestive of concurrent aerocystitis. The animal was treated with injectable antibiotics, anti-inflammatories, and an oral gel diet containing Amprolium for suspected bacterial aerocystitis and myxozoan associated anterior uveitis/scleritis. The fish initially responded well to treatment, with improved appetite and activity, but acutely developed corneal clouding/edema and was found dead 4 weeks later.
Gross Pathology:
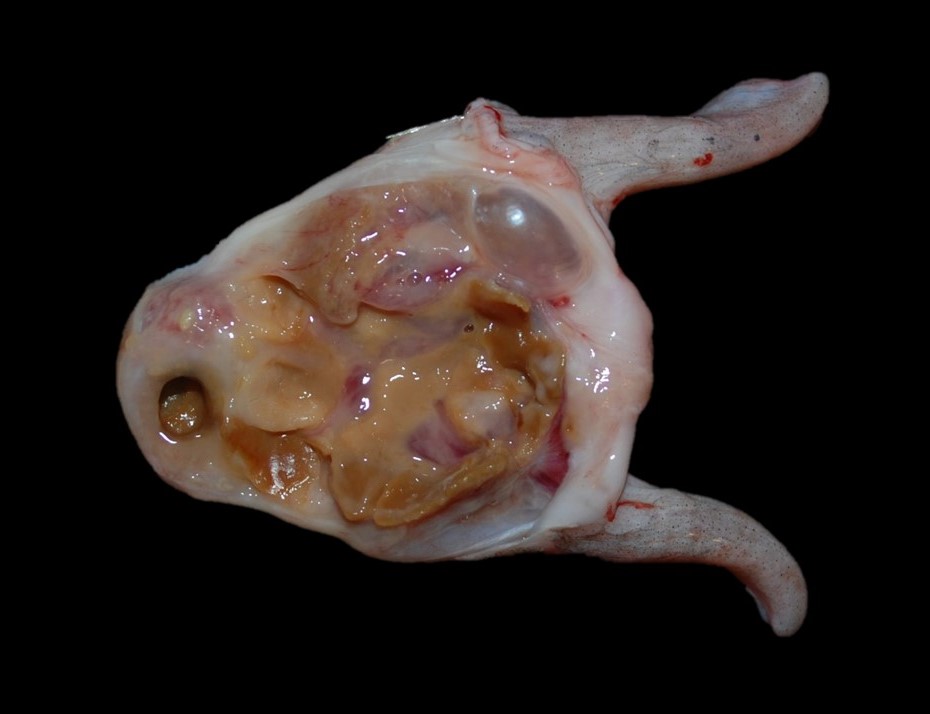
At necropsy, the swim bladder is intact, and the right lobe horn is approximately 20% smaller than the left horn. Adhered to the mucosal surface, and filling the lumen, is abundant, semi-firm, brown to yellow, waxy, material mixed with pale yellow to tan mucoid material and a small amount of red to brown, gelatinous to mucoid material (Fig. 2). Bilaterally, the eyes are mildly shrunken and the corneas are diffusely cloudy. Multifocally within the sclera and bulging onto the peri-ocular surface of the left eye, are five, 0.4 cm to 0.6 cm in diameter, soft, pale yellow nodules, and one 0.5 cm in diameter soft, slightly flocculent dull red nodule. Multifocally within the sclera of the right eye and bulging into the peri-ocular space are six, 0.4 cm to 0.6 cm in diameter, soft, pale yellow nodules, and one 1.0 cm in diameter soft, slightly flocculent dull red nodule.
Laboratory results:
No laboratory findings reported.
Microscopic Description:
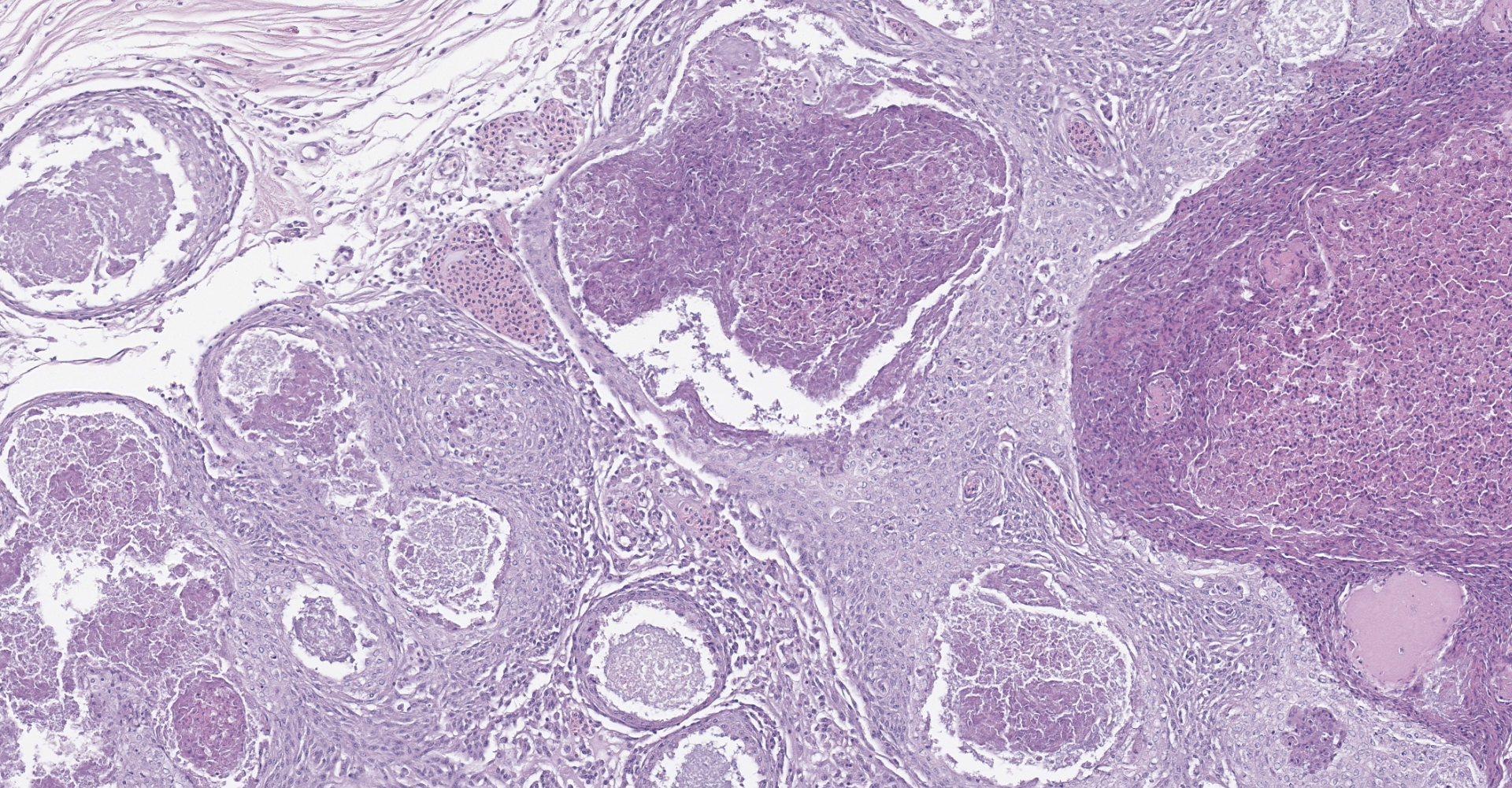
Diffusely, the swim bladder mucosa is markedly expanded and largely effaced by multifocal to locally extensive, variably sized regions of necrosis and inflammation, and increased clear space (edema). Segmentally, the mucosal surface is ulcerated and replaced by a large sheet of epithelioid macrophages
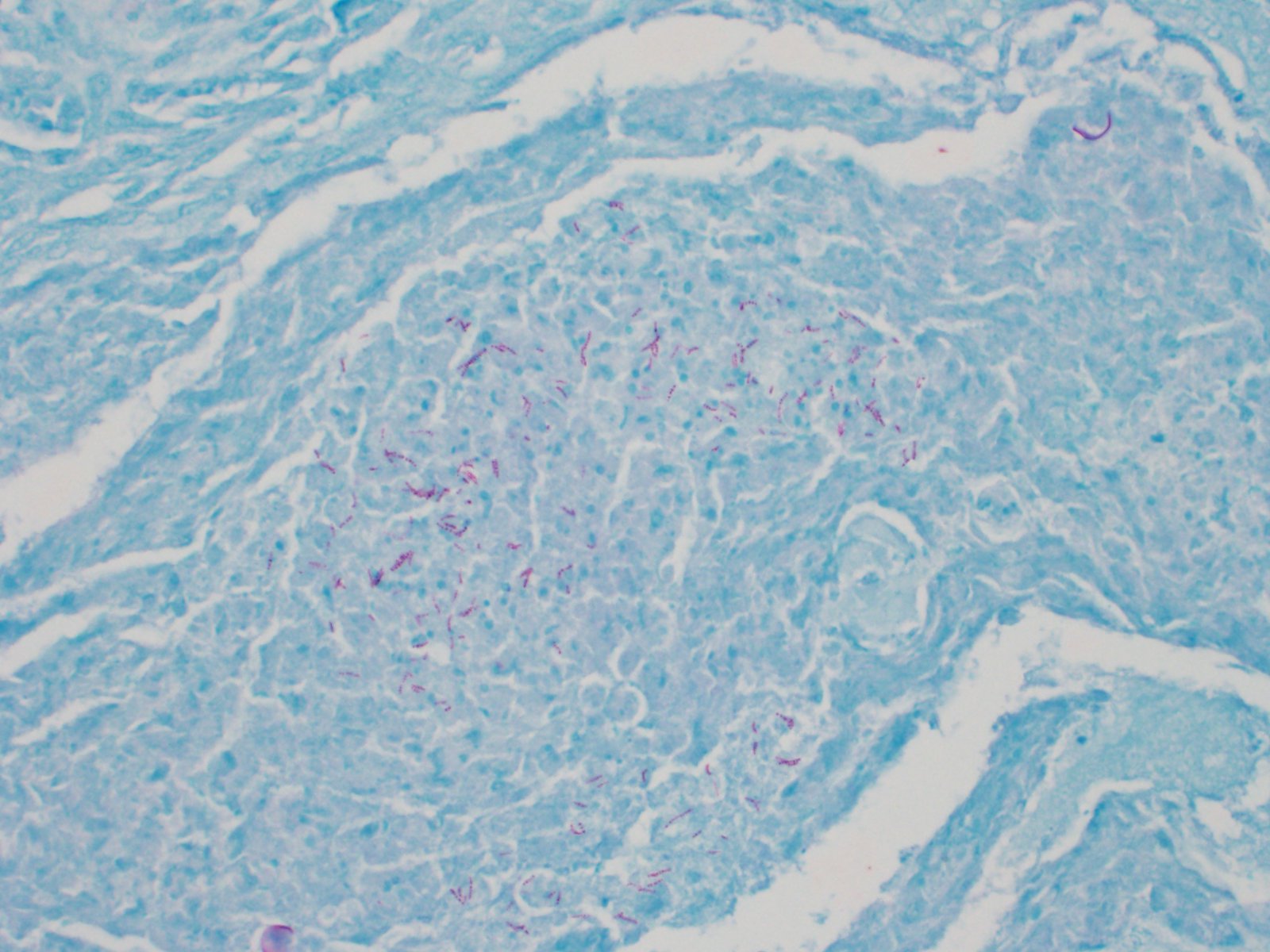
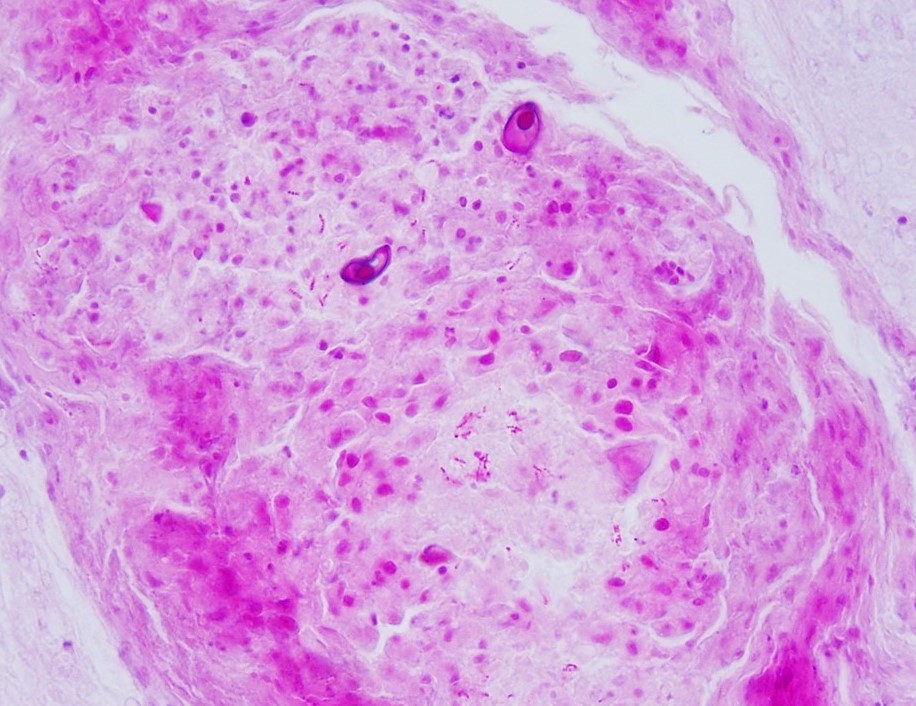
mixed with small numbers of lymphocytes and covered by a thick plaque of brightly eosinophilic necrotic material with pyknotic and karyorrhectic nuclear debris. Within the gas gland and rete mirabile and extending into the submucosa, are multifocal to locally extensive, granulomas with variably thick layers of epithelioid macrophages, central brightly eosinophilic material with pyknotic and karyorrhectic nuclear debris or pale basophilic stippled material, and a peripheral rim of fibroblasts and occasional lymphocytes. Within these regions of necrotic debris as well as in the cytoplasm of intact macrophages are abundant negative staining, thin, bacilli. Also within regions of inflammation and in vessels, are small numbers of myxospores. Spores are ovoid to pyriform, approximately 10-12 um long and 8-10 um wide, with a refractile, 1 um thick wall, two pyriform polar capsules oriented at one pole, and an occasionally visible, 1 um in diameter basophilic nucleus. The gas gland is moderately thickened up to 20-30 cell layers with multifocally hypertrophied epithelium up to 3x the normal cell size. Infiltrating the rete mirabile are small to moderate numbers of lymphocytes and histiocytes. Vessels are multifocally, mildly congested and frequently lined by plump endothelial cells. Scattered throughout the section are mildly increased numbers of rodlet cells. In some sections, there is a large round to oval region of clear space within the rete mirabile (presumed entrapped gas).
Contributor's Morphologic Diagnoses:
Swim bladder, Aerocystitis, granulomatous, necrotizing, multifocal to locally extensive, acute and chronic, severe with mucosal ulceration, edema, gas gland epithelial hyperplasia, and abundant intralesional and intrahistiocytic negative staining bacilli.
Swim bladder, Myxozoa, intravascular and intralesional, small numbers.
Contributor's Comment:
This case represents a chronic mycobacterial swim bladder infection with concurrent systemic myxozoa spread from a primary ocular myxozoan infection.
The swim bladder develops as a diverticulum of the pharynx, with either maintenance of a pneumatic duct between the swim bladder and the pharynx/esophagus in soft-rayed fishes (physostomes) or loss of the pneumatic duct in spiny-rayed fished (physoclistous).3,7,10 The swim bladder's primary role is buoyancy, though it is also thought to play a role in sound reception/production and pressure reception.3,10
The swim bladder is commonly located in the dorsal 1/3 of the coelom, ventral to the posterior kidney and vertebral column, but the anatomy can vary between different species. The swim bladder can be ovoid or multilobulated, and may be one chamber (Salmonidae, Syngathidae) or two chambers (Cyprinidae).3,7,10 Wall thickness can also vary by species from thin and translucent to thick and opaque white. Histologically, the wall of the swim bladder is divided into four layers. The most internal layer, is the mucosa, which is comprised of single layer of cuboidal to transitional epithelium that is responsible for producing surfactant. The second layer is the muscularis, which can be composed of smooth muscle or skeletal muscle depending on the species, followed by the submucosa, which is composed of loosely arranged fibroblasts and collagen fibers that is also highly vascular. The most external layer is the external tunic, which is composed of compact fibroblasts and collagen bundles and a thin serosa.3,10 There are three functional components to the swim bladder: the oval, the gas gland, and the rete mirabile.3 The oval is on the caudodorsal wall of the swim bladder, and helps regulate oxygen movement from the lumen into the blood to reduce swim bladder volume.3 The gas gland is located on the cranioventral aspect of the swimbladder and consists of a variably thick epithelial layer of gas gland cells responsible for acidifying the blood and releasing oxygen into the lumen.2 Blood supply to the gas gland is provided by the rete mirabile, which is composed of many arterial and venous capillaries allowing for countercurrent blood flow.3
In the swim bladder of this guineafowl puffer, there was severe granulomatous and necrotizing inflammation with abundant intralesional acid-fast bacilli consistent with a Mycobacterium spp. Carnobacterium maltaromaticum and Aeromonas hydrophila were also cultured from the swim bladder, but these were considered a secondary infection and were not histologically apparent.
Mycobacteriosis in fish often presents as a chronic disease for which clinical signs may not be observed. 4 Multiple non-tuberculous Mycobacterium species have been reported to cause disease in fish, though M. marinum, M. chelonea and M. fortuitum are reported as the most common isolates.4 Systemic dissemination to multiple organs is common in fish mycobacteriosis, and associated inflammation is often granulomatous. Lesions can range from a mixture of macrophages and occasional granulocytes in acute lesions, to discrete granulomas with epitheloid macrophages centrally and a periphery of fibroblasts and lymphocytes in more chronic lesions.
This fish also had a severe, bilateral ocular myxozoan infection, and on histopathologic examination, myxospores were noted in the swim bladder, vessels in the wall of the gastrointestinal tract, and melanomacrophage centers in the spleen and anterior kidney, supporting systemic spread. The myxozoa were not considered to be a primary cause of inflammation in the swim bladder, and likely colonized the region opportunistically. At the time of submission, a definitive identification of the myxozoan was not available; however, based on the cytologic appearance of the organisms and histologic lesions centered on the scleral cartilage in the eyes, a Myxobolus spp. was considered most likely.
While some species of myxozoans, such as Myxobolus cerebralis, Henneguya ictaluri, and Tetracapsuloides bryosalmonae, are known to cause significant clinical disease, many infections are subclinical.1,6 However, if myxozoan cysts rupture, the release of the spores and can incite a local inflammatory response and occasionally involve other organ systems.1,6 Presumably, this fish arrived into quarantine with a subclinical ocular myxozoan infection, and it was not until the myxozoan cysts ruptured that clinical signs and grossly apparent ocular lesions manifested.
Contributing Institution:
Wildlife Conservation Society
2300 Southern Blvd.
Bronx, NY 10460
JPC Diagnosis:
1. Swim bladder, mucosa and submucosa: Aerocystitis, granulomatous, multifocal to coalescing, severe, with ulceration, intravascular gas bubble formation, rodlet cell hyperplasia, and gas gland hyperplasia and hypertrophy.
2. Swim bladder, granulomas and rete mirabile: Myxospores, numerous.
JPC Comment:
The contributor provides an excellent review of the development, anatomy, physiology, and roles associated with the swim bladder in various fish species as well as an overview of mycobacteriosis and myxozoans.
Mycobacteriosis in fish was first described in carp (Cyprinus carpio) by Bateaillon et al. in 1897. As noted by the contributor, three Mycobacterium spp. have since dominated the literature in regard to fish: Mycobacterium marinum, Mycobacterium fortuitum, and Mycobacterium chelonae.4
Resistant to the normal processes of acidification and phagolysosomal fusion, pathogenic mycobacteria are predominately intracellular organisms found within phagocytes. Within mammalian macrophages, pathogenic mycobacteria are thought to retard phagosome development and formation of the phagolysosome by preventing fusion with components of the late endosomal network. Furthermore, M. marinum in mouse macrophages has been shown to escape phagosomes and spread directly from cell to cell via actin-based motility. However, less information exists in regard to the behavior of mycobacteria within the macrophages of poikilotherms. Bearing resemblance to what is classically observed in mammalian species, M. marinum was found to inhibit phagolysosome fusion when using cell lines derived from carp species. However, studies have also shown phagolysosome fusion occurs in vivo in striped bass infected with M. marinum, with similar findings in frog and fish tissues. Therefore, it is likely M. marinum, and possibly other bacteria within the same genus, employ multiple intracellular survival strategies in poikilotherms.4
A recent report described a continuum of variably mature granulomatous lesions caused by Mycobacterium chelonae in two farmed Japanese pufferfish (Takifugu rubripes) within the swim bladder, kidney, spleen, and gills.5 The most mature lesions were encapsulated granulomas with central necrosis that were most commonly identified in the swim bladder. Intermediate lesions with encapsulated granulomas without central necrosis and immature lesions with unencapsulated clusters of epithelioid macrophages were most frequently identified in the kidney and spleen, and the gills most commonly exhibited immature lesions. The presence mature granulomas in the swim bladder in comparison to other locations suggested M. chelonae induced lesions in the swim bladder either formed earlier during the infection due to increased tissue susceptibility or M. chelonae induced lesions developed more rapidly in the swim bladder compared with other organs. Given that the swim bladder is composed primarily of oxygen, the authors of the report suggested the association of the mature lesions with the swim bladder may have been due to the typically aerobic nature of Mycobacterium spp., as well as the organ's connection to the circulatory system with a rich blood flow.5
In this case, acid fast stains (Fite-Faraco and Ziehl-Neelsen) reveal small numbers of filamentous acid-fast bacilli within multifocal granulomas. Although these findings are consistent with Mycobacterium spp., results of confirmatory testing such as PCR, if performed, were not included in the WSC submission.
Myxozoans are metazoan parasites with characteristic polar capsules and coiled polar filaments along the inner wall of the capsule, which are essential to initiating host infection when discharged. Myxozoans predominantly infect poikilothermic animals, with over 700 species described in fish. These parasites have a complex life-cycle that often alternates between vertebrate and invertebrate hosts with sporogony occurring in each. As noted by the contributor, the majority of infections cause chronic subclinical disease with little to no clinical signs or host immune response. However notable exceptions associated with high morbidity and mortality and significant economic loss include Myxobolus cerebralis, Henneguya ictaluri, and Tetracapsuloides bryosalmonae, the species associated with whirling disease of the salmon, proliferative gill disease of the catfish, and proliferative kidney disease of salmon, respectively. Although predominantly considered a disease of farmed fish, whirling disease has been found in wild stock, raising concerns in regard to population decline.1
In addition to multiple layers of epitheliloid macrophages and lymphocytes, the moderator also noted the presence of rodlet cells within the walls of the granulomas. Originally thought to be parasites when first described, rodlet cells are uniquely found in telosts and are considered a component of a generalized host response to various stressors, particularly parasites, but also toxins, neoplasia, and generalized trauma. Rodlet cells contain multiple longitudinal club-shaped sacs (i.e. rodlets) with a protein rich core. Following a one-way contraction of the cell, rodlets are forcefully ejected into the extracellular space or environment, along with the rest of the cell's contents, resulting in its destruction. Following their expulsion, rodlets appear to be passive and stable in the environment but are thought to play a role in combating infection. In addition, these cells are frequently observed in multiple organs, including the skin, liver, heart, spleen, gills, kidney, and the endothelium lining blood vessels. Multiple theories exist in regard to the function of these cells, including the regulation of pH, lubrication, antimicrobial effects, osmoregulation and non-specific immunity.8
References:
1. Cavin JM, Donahoe SL, Frasca S, et al. Myxobolus albi infection in cartilage of captive lumpfish (Cyclopterus lumpus). JVDI. 2012;24(3):516?524.
2. D'Aoust BG. The role of lactic acid in gas secretion in the teleost swimbladder. Comp Biochem Physiol. 1970;32: 637?668.
3. Fange R. Physiology of the swimbladder. Physiol Rev.1966;46:299?322.
4. Gauthier DT and Rhodes MW. Mycobacteriosis in fishes: A review.The Vet Jour.2009;180(1): 33-47.
5. Ishii Y, Kawakami H, Mekata T, Sugiyama A. Histopathological Features of Mycobacterium chelonae Infection in Two Farmed Japanese Pufferfish (Takifugu rubripes). J Comp Pathol. 2019;170:86-90.
6. Kent, ML, Andree, KB, Bartholomew, JL Recent advances in our knowledge of the Myxozoa. J Eukaryot Microbiol. 2001;48:395?413.
7. Lumsden, JS. Gastrointestinal tract, swim bladder, pancreas and peritoneum. In: Ferguson HW, ed. Systemic Pathology of Fish. London, UK: Scotian Press; 2006: 169-199.
8. Mokhtar DM, Abdelhafez EA. An overview of the structural and functional aspects of immune cells in teleosts. Histol Histopathol. 2021;36(4):399-414.
9. Roberts, RJ. 2012. The anatomy and physiology of teleosts. In: Roberts RJ, ed. Fish Pathology, 4th ed. West Sussex, UK: Blackwell Publishing, Ltd; 2012:17-61.
10. Roberts, RJ. 2012. The pathophysiology and systematic pathology of teleosts. In: Roberts RJ, ed. Fish Pathology, 4th ed. West Sussex, UK: Blackwell Publishing, Ltd; 2012: 62-143.