CASE 3: S 106/18 (4119806-00)
Signalment:
Eight month old, intact, male Hanoverian horse (Equus ferus caballus)
History:
The horse originally came from Poland. The colt was diagnosed with pneumonia due to suspected infection with Rhodococcus equi and received azithromycin, tulathromycin and rifampicin as antibiotic treatment. Shortly after treatment the animal presented in the equine clinic with signs of colitis including fever, profuse diarrhea, dehydration and severe hypoproteinemia, which was nonresponding to any kind of treatment. The colt received intensive medical care including plasma transfusions, constant infusion therapy (e.g. glucose, sodium bicarbonate, Ringer's solution), probiotic treatment. A fecal culture detected Klebsiella pneumoniae and Clostridium difficile. The colt was euthanized shortly afterwards due to therapeutic failure and with pure prognosis.
Gross Pathology:
The young stallion was in a very poor nutritional condition with signs of early serous atrophy of the cardiac, coronary, and the bone marrow fat. Severe subcutaneous edema affected the head, the ventral aspect of the neck and the ventral abdomen.
Two focal abscesses were detectable in the lung, which measured about 0.5 cm and 0.8 cm in size respectively. In addition, there was severe acute diffuse alveolar edema and severe acute diffuse congestion of the lung. In the abdominal cavity there was moderate serofibrinous ascites (6 liters).
The wall of the stomach was multifocal markedly thickened (1.8 cm) by a predominantly submucosal edema. The mucosa itself was hyperemic but revealed no signs of ulceration or erosion.
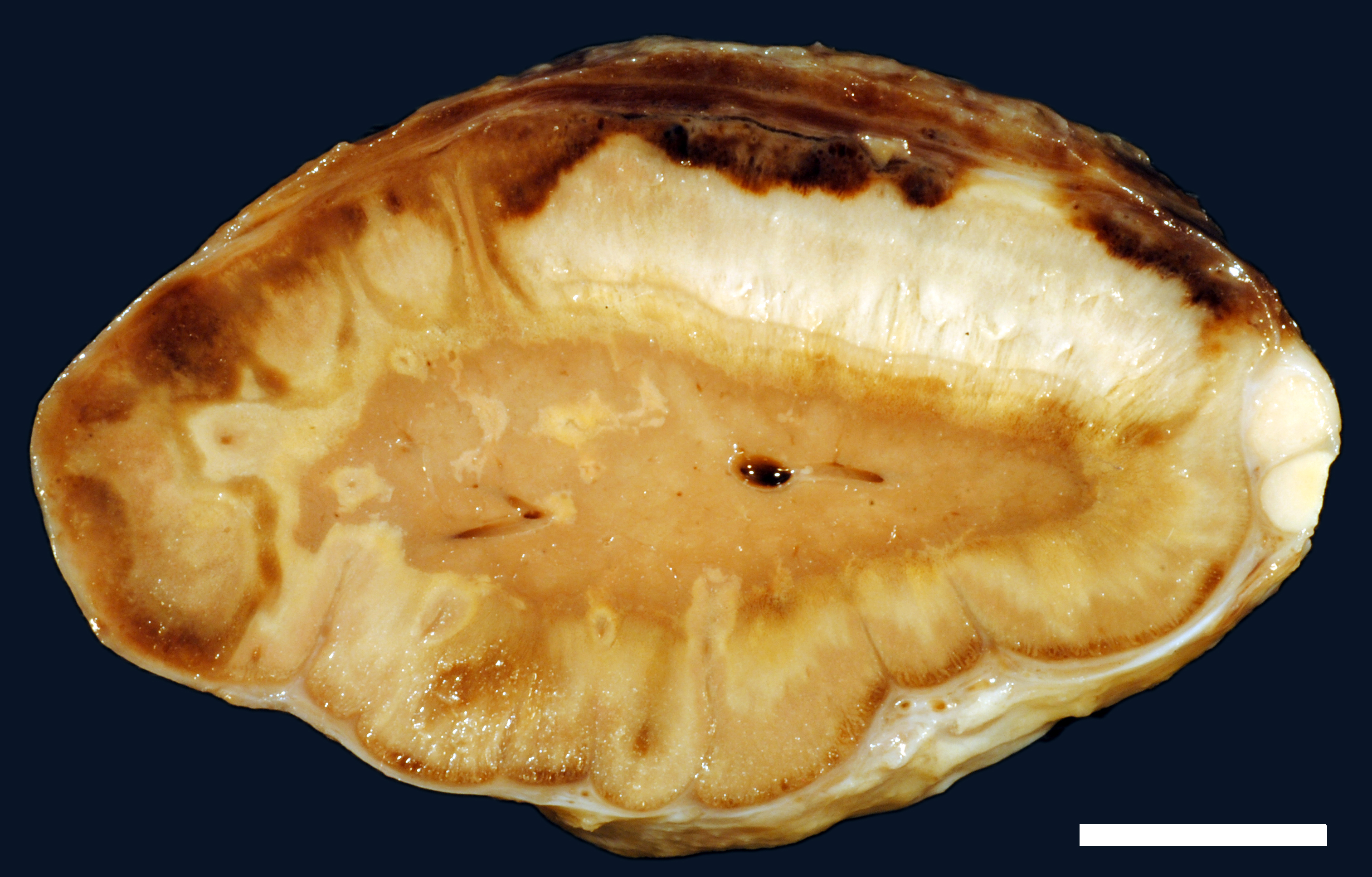
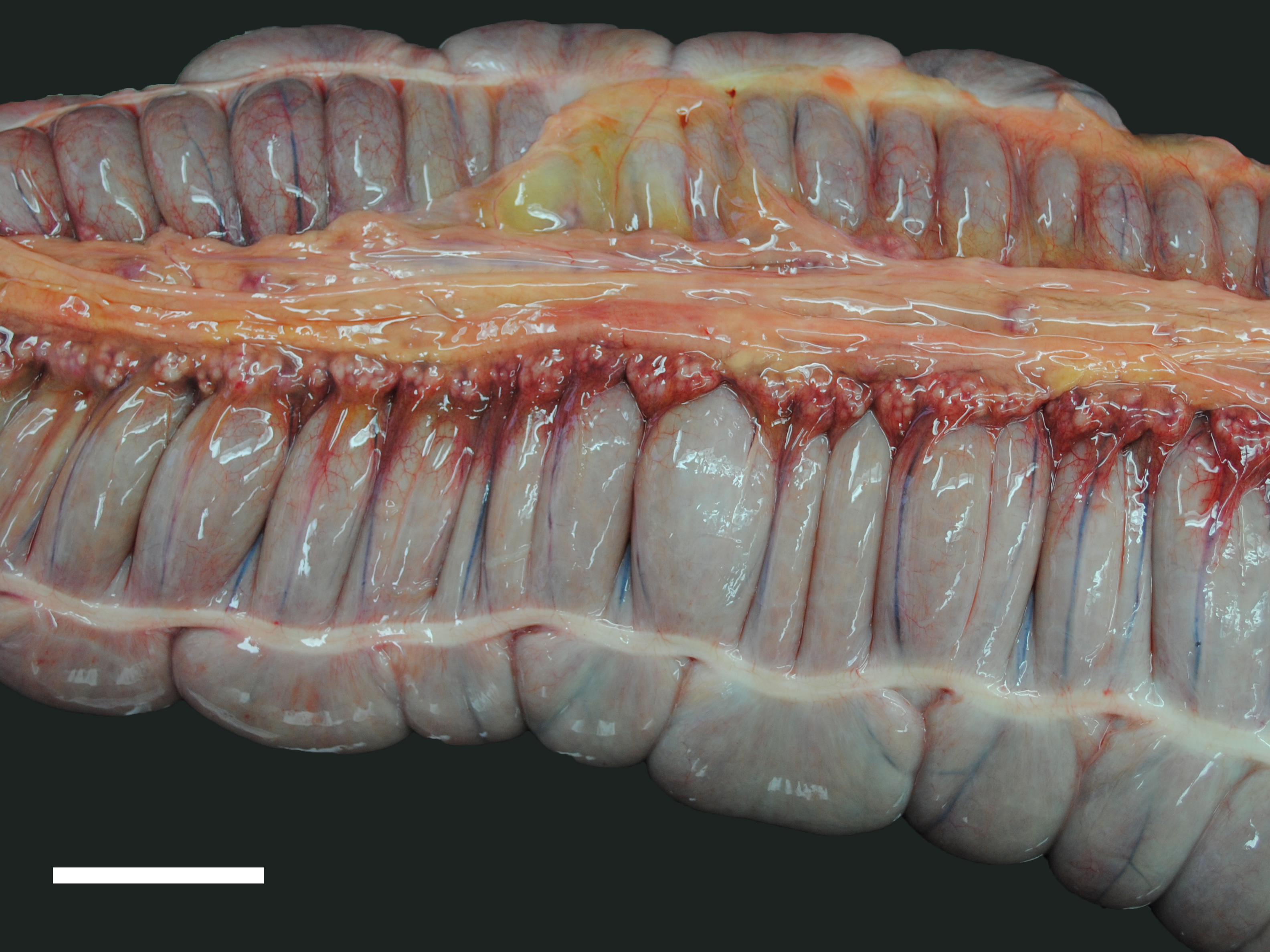
A moderate number of adult nematodes, morphologically consistent with Strongylus spp., was detected in the small intestine. The large intestine was filled with green fluid with only little, finely structured particles. The intestinal wall was diffusely thickened and edematous (max. 1.8 cm) and there were multifocal mucosal erosions. In the mesocolon multifocal petechial and ecchymatous hemorrhage was present. The regional lymphatic tissue was moderately hyperplastic. The adrenal glands had multifocal to coalescing areas of pale (necrosis) and reddish (hemorrhage) discoloration.
Laboratory results:
Microbiology:
See chart.
Microscopic description:
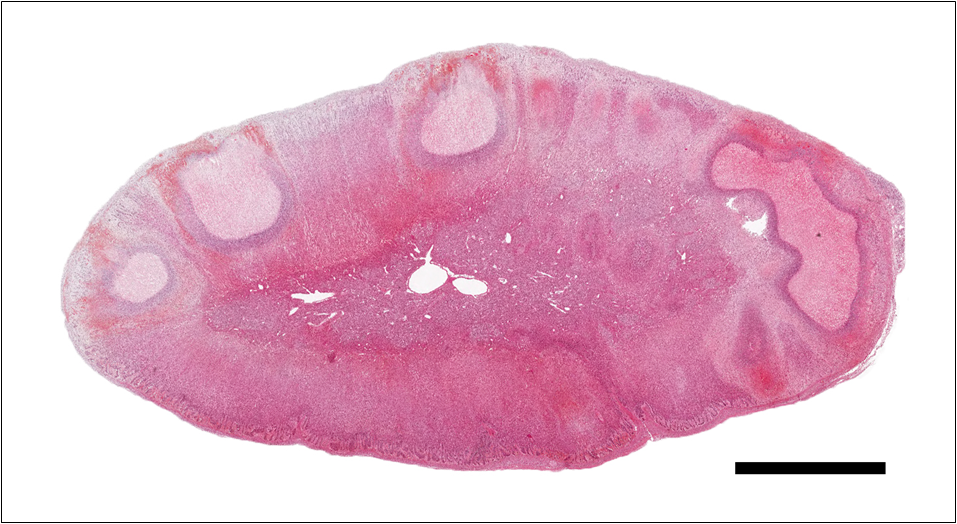
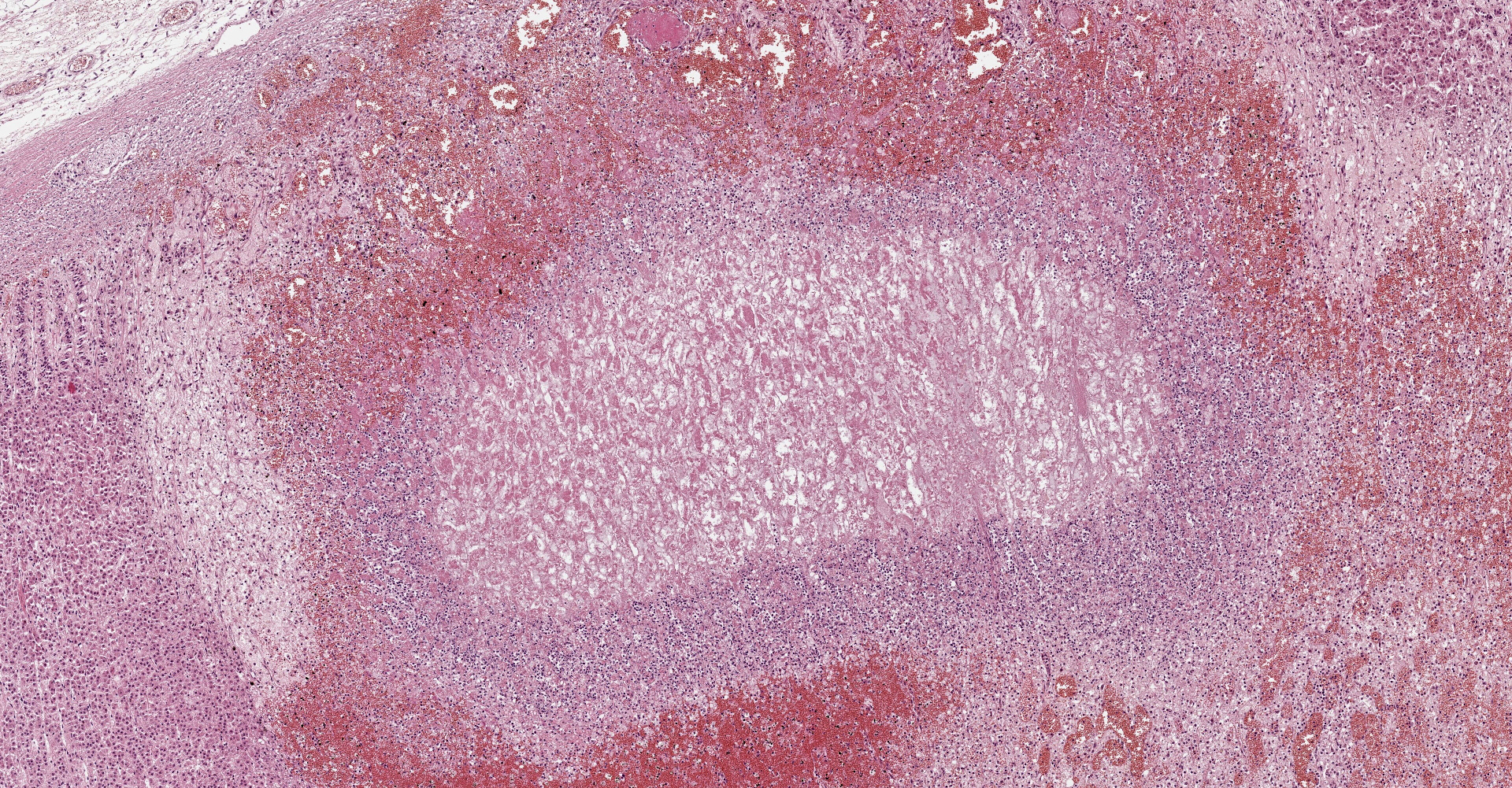
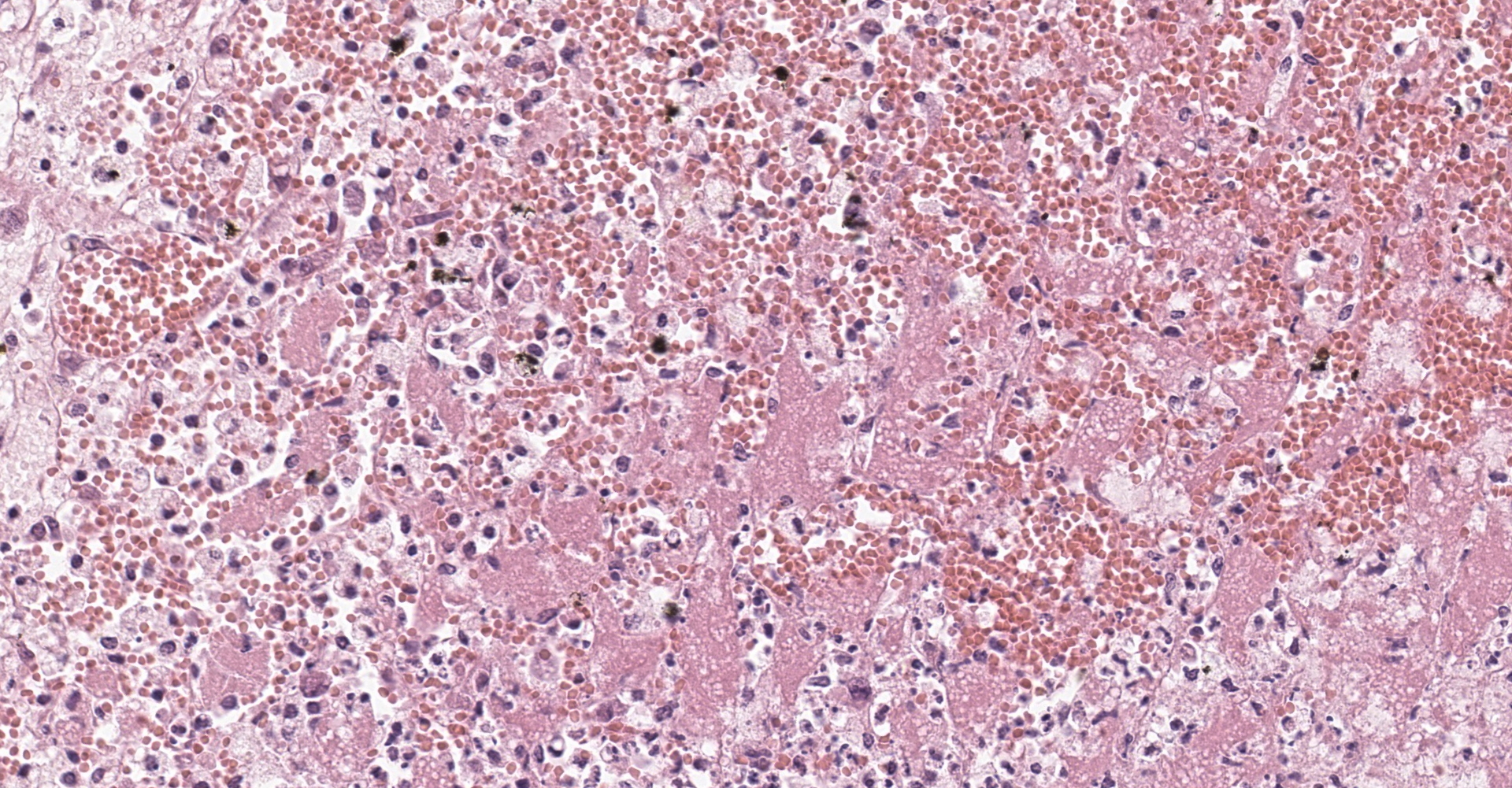
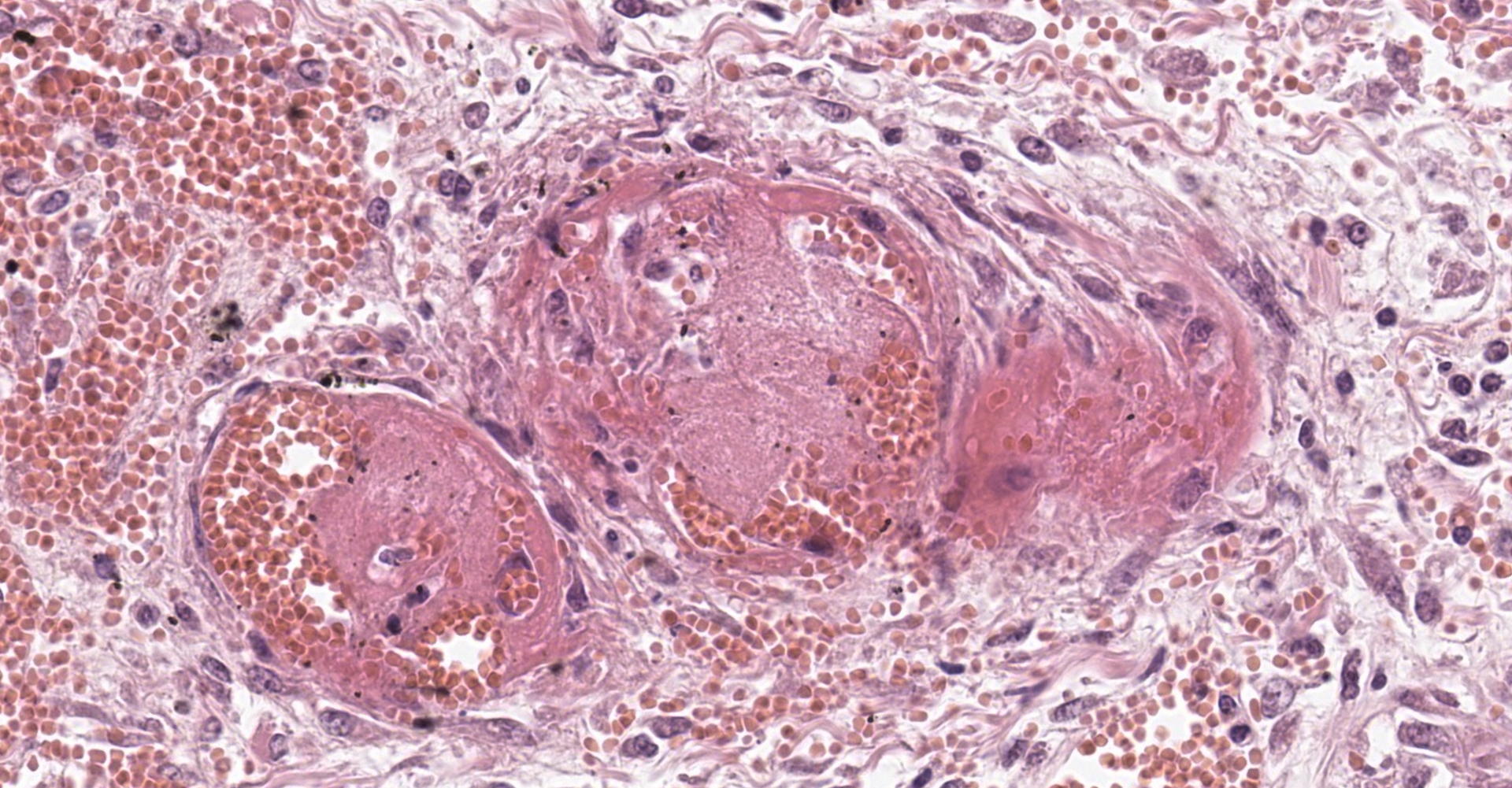
Adrenal gland: Within the adrenal cortex there are multifocal widespread areas with loss of cellular detail and differential staining (coagulation necrosis) surrounded by a rim of high numbers of degenerated neutrophils. In neighbored areas there are widespread accumulations of extravasated erythrocytes (hemorrhage) as well as multifocal small vessels occluded by homogeneous, eosinophilic material (fibrin thrombi). The adrenal medulla was unchanged.
Contributor's morphologic diagnosis:
Adrenal gland: Cortical necrosis, marked, multifocal to coalescent, acute with hemorrhage, moderate, multifocal, acute
Contributor's comment:
Acute, severe adrenal hemorrhage is also termed adrenal apoplexy. It is a well- known condition in human and veterinary medicine and often associated with bacterial infections.4 The most frequent clinical condition associated with infectious adrenal hemorrhage in human patients is an endotoxin-mediated septic shock.3 Under the specific circumstances of adrenal hemorrhage in association with sepsis the condition is also named Waterhouse-Friderichsen Syndrome.8 The most common and first described pathogens leading to Waterhouse-Friderichsen Syndrome in children were meningococci, but other bacteria such as Streptococcus spp., Staphylococcus spp., Clostridium spp., Klebsiella spp. and others have been identified as possible agents.3
Especially in the adult horse, adrenal hemorrhage is often associated with acute, severe gastrointestinal diseases.4 In this specific case of antibiotic-induced colitis, Klebsiella pneumoniae as well as Clostridium difficile were detected in different organs including the large intestine and intestinal lymph nodes. Within the adrenal gland, no bacterial structures were detectable using the Gram and the Giemsa stains, which supports the hypothesis of endotoxin-induced adrenal hemorrhage.
In humans the arteries supplying the adrenal gland are branched 50-60 times. These smaller arteries open into relatively straight capillaries which are only passing through the zona fasciculata and abruptly form a vascular plexus around the zona reticularis.2 This plexus seems predisposed to vascular damage, and the pattern of necrosis and the localization of the fibrin thrombi make such a pathogenesis probable, even in the present case.
Another predisposing factor, mentioned by several authors, is the hyperactivity of the adrenal cortex, particularly the increased synthesis of corticosteroids or at least an increased level of adrenocorticotropic hormone (ACTH) seems to raise the susceptibility of the adrenal cortex to endotoxin-induced hemorrhage.3,8
Contributing Institution:
Department of Veterinary Pathology
Freie Universita?t Berlin
http://www.vetmed.fu-berlin.de/en/einrichtungen/institute/we12/index.html
JPC diagnosis:
Adrenal gland, cortex: Necrosis, coagulative and lytic, multifocal to coalescing, with venous thrombosis and hemorrhage.
JPC comment:
This condition was described in calves more than 40 years ago, most often as sequela from bacterial sepsis. In these cases, there was multifocal hemorrhage and necrosis in the cortex, specifically in the zona glomerulosa and the inner parts of the zona fasciculata. In most cases, fibrin thrombi and platelet aggregates were observed in capillaries and sinusoid veins of the zona glomerulosa and the zona fasciculata. In areas of necrosis, large, individualized globules (10-20 mm) of fibrin were observed between cortical cells.5
In humans, this condition occurs most frequently in children, though this lesion is seen in approximately 1% of routine autopsies.7 This syndrome has also been reported in nonhuman primates, including a female gang-housed baboon1, as well as in a cynomolgus monkey with staphylococcal meningitis.9 A recent investigation of systemic inflammatory response syndrome (SIRS) in pig tailed macaques (Macaca nemestrina) and yellow baboons (Papio cynocephalus) found bilateral adrenal hemorrhage (Waterhouse-Friderich syndrome) was a common sequela, in addition to multi-organ dysfunction (MOD) or failure, acute respiratory distress syndrome (ARDS), and disseminated intravascular coagulation (DIC).6
The adrenal lesion presented here may be frequent in humans but is not a common diagnosis in horses. This case bears similarities with human cases but may have a slightly different pathogenesis. This syndrome was observed in nonhuman primates as a sequela to septicemia and did not develop during the initial insult. The fibrin thrombi observed in this case appear to be in veins, with no arteries in section occluded. While this appears to largely be the result of arteriolar compromise and ischemia, it is not evident in this section. With respect to the laboratory test results, the pattern of necrosis is considered less likely to be the result of C. difficile but may be consistent with the detected Klebsiella pneumoniae.
Accessory adrenal cortical tissue is an incidental finding seen in a large proportion of horses and does not play a role in the pathogenesis of this disease.
References:
- Cary M, Kosanke S, White G. Spontaneous Waterhouse-Friderichsen syndrome in a gang-housed baboon. J Med Primatol. 2001;30:185-187.
- Edwards OM. Adrenal apoplexy?the silent killer. J R Soc Med. 1993;86(1):1? 2.
- Guarner J, Paddock CD, Bartlett J, Zaki SR. Adrenal gland hemorrhage in patients with fatal bacterial infections. Modern Pathology 2008;21:1113-1120.
- Hart KA, Barton MH. Adrenocortical Insufficiency in Horses and Foals. Vet Clin North Am Equine Pract. 2011;27(1):19-34.
- Hoffman R. Adrenal lesions in calves dying from endotoxin shock, with special reference to the Waterhouse-Friderichsen Syndrome. J Comp Path. 1977;87:231-239.
6. Hukkanen RR, Liggitt HD, Murnane RD, Frevert CW. Systemic inflammatory response syndrome in non-human primates culminating in multiple organ failure, acute lung injury, and disseminated intravascular coagulation. Toxicol Pathol. 2009; 37(6): 799-804.
7. Karki BR, Sedhai YR, Bokhari SRA. Waterhouse-Friderichsen Syndrome. [Updated 2020 Jun 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551510/
- Levin J, Cluff LE. Endotoxemia and adrenal hemorrhage. A mechanism of the Waterhouse-Friderichsen Syndrome. J Exp Med. 1965; 121(2):247-260.
- Lin Z, Zhang L, Zhang D, et al. A case of spontaneous staphylococcal meningitis in a cynomolgus monkey. J Med Primatol. 2018;47(2):132-135.