WSC 2023-2024, Conference 13, Case 3
Signalment:
14-week-old female Sprague Dawley rat (Rattus norvegicus)
History:
This animal gave birth with no apparent complications. At seven days post-partum, she developed paraparesis of both hind limbs. No history of trauma or mishandling was noted by the laboratory.
On physical exam, the rat was bright, alert, and responsive, euhydrated, and nursing her pups. Neurologic exam revealed absent conscious proprioception of both hind limbs, but motor reflex was intact when stimulated with a toe pinch. There was no overt pain on spinal palpation, and urination and defecation were reported to be normal. Over 48 hours, paraparesis progressed, and she lost ~7% of body weight. Euthanasia was elected due to poor prognosis.
Gross Pathology:
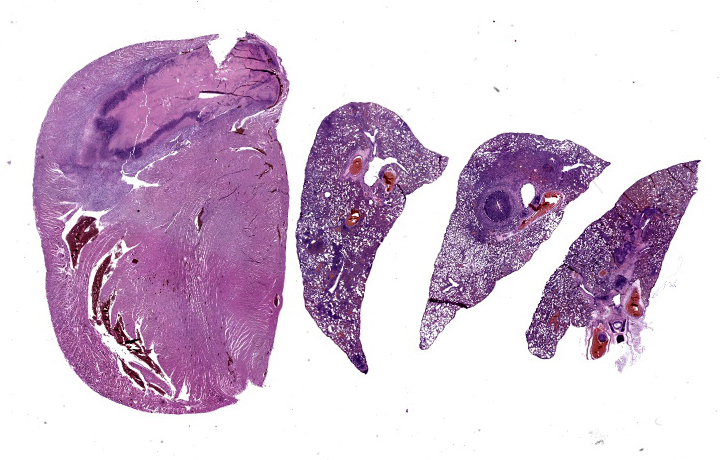
On necropsy, the lungs were diffusely pink with multifocal dark pink depressions and all sections floated in formalin. The heart was diffusely dark red. On cut section, a 2x5x2 mm thrombus was adhered to the right atrioventricular valve. Both kidneys were diffusely dark red and unremarkable on cut section. The spine/spinal cord appeared grossly unremarkable.
Laboratory Results:
PCR results on formalin-fixed cardiac tissue:
Corynebacterium kutscheri: Negative.
Staphylococcus aureus: Negative.
Streptococcus pneumoniae: Negative.
Group B Streptococcus (GBS): Positive.
Microscopic Description:
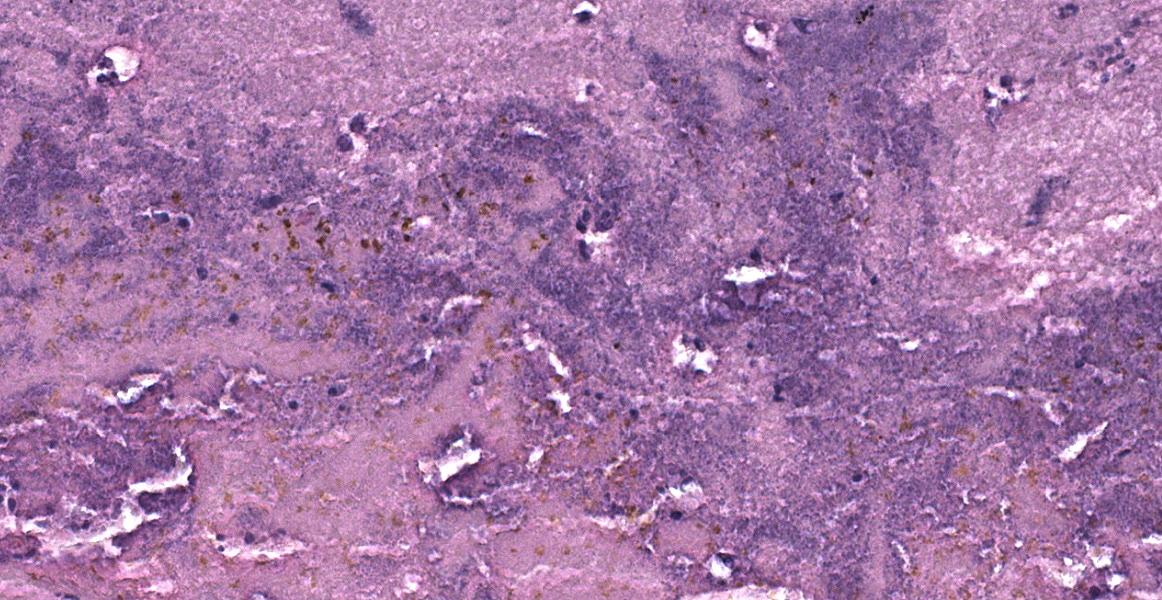
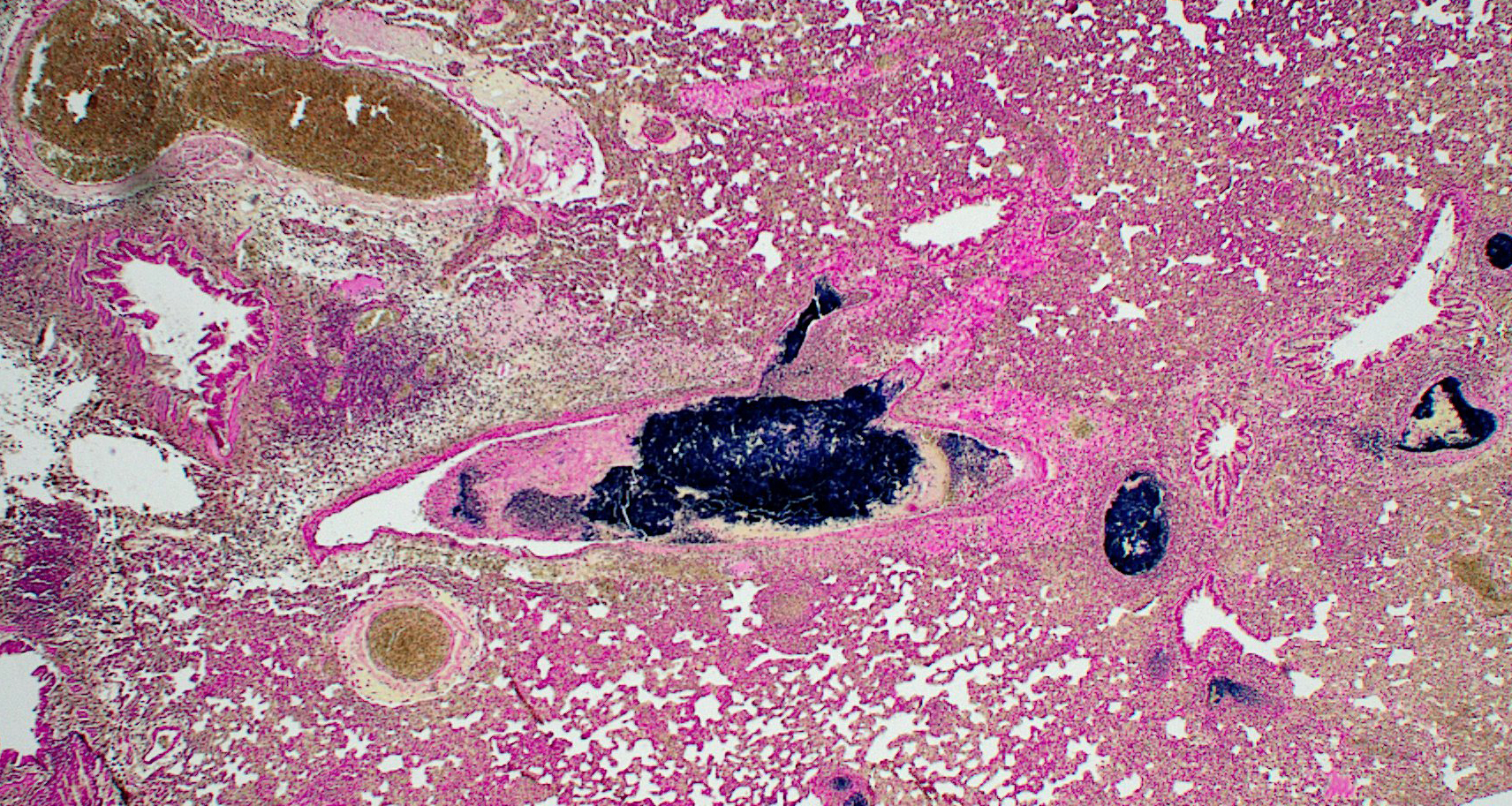
Lung: Approximately 50% of small, medium, and large-caliber pulmonary vessels are distended and occluded by intralumenal abundant degenerate neutrophils, karyorrhectic debris, eosinophilic fibrillar material (fibrin), and large basophilic bacterial colonies (thrombi/thromboemboli). The elastic laminae are frequently indistinguishable, and the tunica intima, media, and adventitia are replaced by abundant degenerate neutrophils and karyorrhectic debris (necrosis). There is moderate perivascular edema and perivascular inflammation comprised of moderate numbers of neutrophils, macrophages, and lymphocytes. Occasionally, alveolar septa are expanded up to 5 times normal width by variable numbers of neutrophils, lymphocytes, and histiocytes. Bronchi/bronchioles are unremarkable.
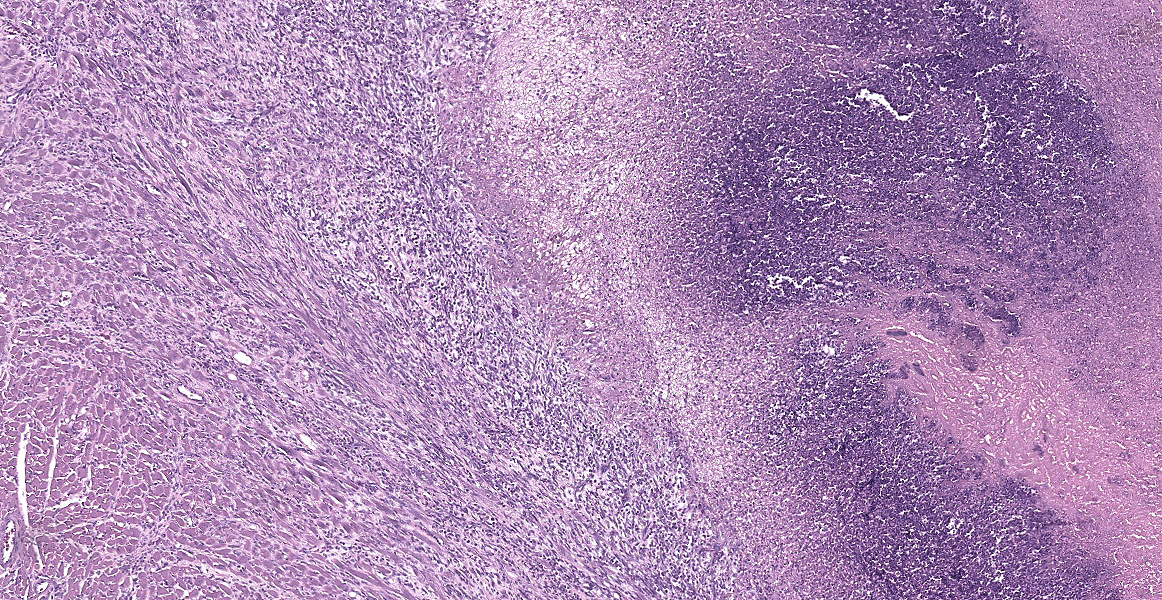
Heart: The endocardium and right atrioventricular valve are expanded and replaced by large numbers of plump spindled cells (fibroblasts) associated with parallel capillaries within a lightly eosinophilic, fibrillar (collagenous) matrix (granulation tissue). Attachedto the endocardium is a thick layer of degenerative neutrophils and karyorrhectic debris with a central area of brightly eosinophilic amorphous to laminated material (thrombus) and coalescing large basophilic bacterial colonies. Infiltrating the myocardium and dissecting around myofibers are proliferating fibroblasts and multifocal mixed inflammation consisting of neutrophils, macrophages, and lymphocytes. There is mild myofiber degeneration characterized by cytoplasmic hypereosinophilia, swelling, loss of striations, and pyknotic nuclei. The epicardium is focally expanded by mild mixed mononuclear infiltrates at the apex of the heart.
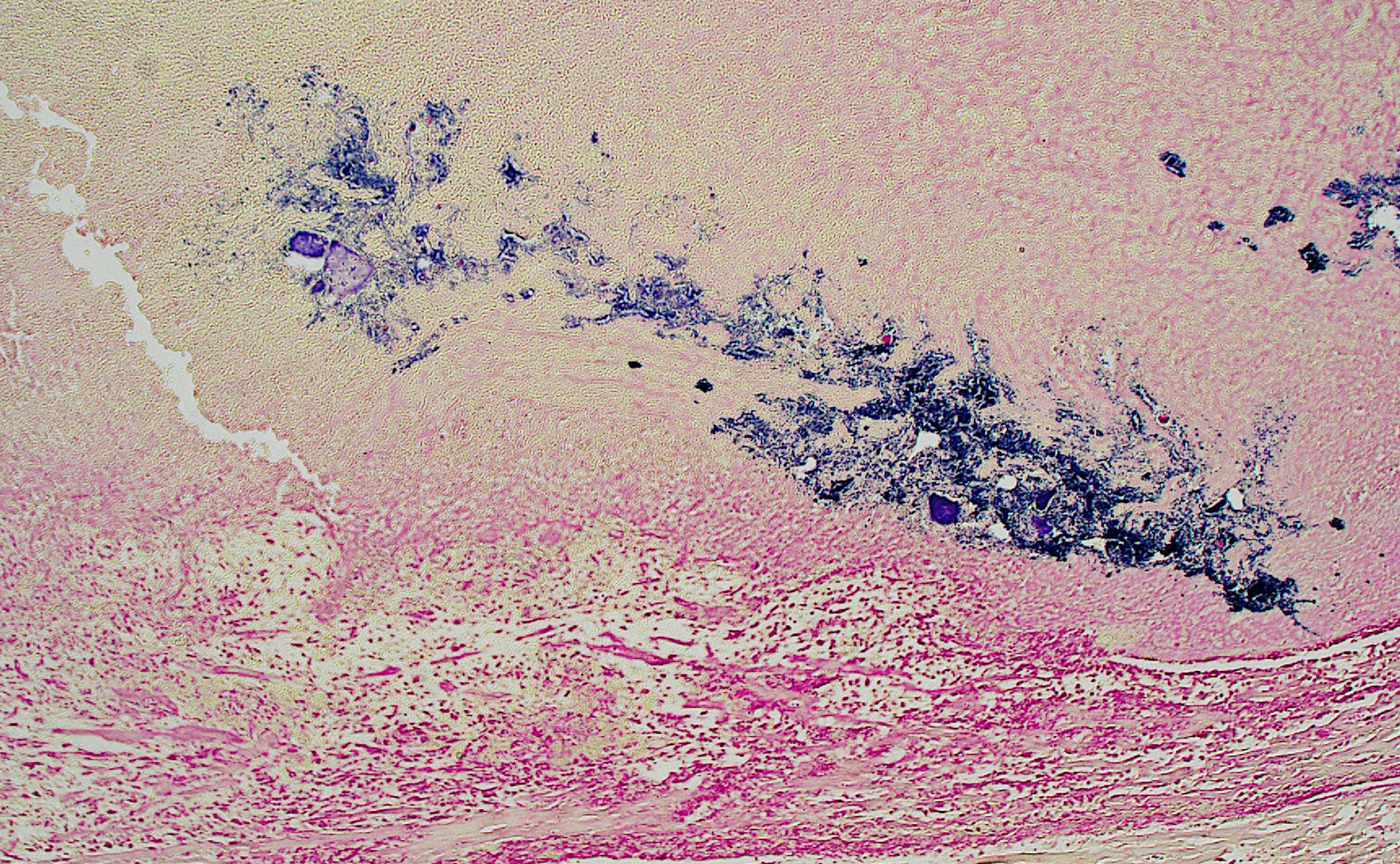
Gram staining of the lung revealed abundant intravascular Gram-positive bacterial diplococci. Gram staining of the heart revealed abundant Gram-positive bacterial cocci within an atrial thrombus.
Contributor’s Morphologic Diagnoses:
1. Lung: Obstructive thromboemboli, chro-nic, severe with necrosuppurative vasculitis/perivasculitis, and intravascular large bacterial colonies (cocci).
2. Lung: Interstitial pneumonia, chronic, multifocal to coalescing, severe, neutrophilic and lymphohistiocytic, with intralesional large bacterial colonies (cocci).
3. Heart, right atrium and AV valve: Endocarditis and myocarditis, fibrinosuppurative, chronic, regionally-extensive, severe with intralumenal thrombus, large colonies of bacterial cocci, and granulation tissue formation.
Contributor’s Comment:
In addition to the right atrial thrombus and embolic bacterial pneumonia, significant microscopic findings include severe suppurative inflammation in the bladder, kidneys, and right stifle joint. Clinically noted ‘paraparesis’ is attributed to septic arthritis in the right stifle, though thromboemboli affecting the spinal cord or other sites is also possible.
In laboratory rodents, expected causes of prominent intralesional bacterial colonies associated with pneumonia can include Corynebacterium, Streptococcus, and Staphylococcus sp. Other bacterial causes of pneumonia in rats include Pseudomonas aeruginosa (gram-negative), Mycoplasma pulmonis (gram-negative), and Filobacterium rodentium (gram-negative).2,3,8
Important features of this case include a prominent embolic pattern without significant involvement of the large and small airways. A major differential for bacteremia and embolic lesions in rats is Corynebacterium kutscheri (pseudotuberculosis), a gram-positive short rod that can cause similar changes to this case.3,4,8
Gram stain of the heart and lungs revealed intravascular gram-positive pairs and chains of cocci in thrombi, consistent with streptococcal infection. Consistent with non-aerogenous extra-pulmonary bacterial etiology, bacteria were not evident in airways. PCR on formalin-fixed tissues was positive for Group B Streptococci (GBS).
GBS are commensal bacteria residing in the urogenital, respiratory, and intestinal tract and can be associated with opportunistic infections, particularly in neonates and immunosuppressed individuals.6 Most notably, maternal colonization of GBS in humans is a risk factor for neonatal meningitis, septicemia, pneumonia, and death. However, GBS infection in gravid rats is reported to cause multi-systemic lesions (suppurative pan-carditis, splenitis, pancreatitis, interstitial pneumonia, metritis, and bacterial thromboemboli) in the dam, with suppurative urocystitis as the possible source.3 Uterovaginal origin also seems likely post-partum, however these tissues were unremarkable in our case.
GBS were among the most commonly identified bacteria in rat specimens (2016-2020 PCR results) in a recent report, with approximately 20% prevalence.1 FELASA 2014 recommends quarterly monitoring for GBS in rats.7 GBS is frequently excluded from routine health monitoring in laboratory rodents.5 This case highlights a potentially important cause of morbidity in breeding colonies.
Contributing Institution:
Johns Hopkins University
School of Medicine
Department of Molecular & Comparative Pathobiology
https://mcp.bs.jhmi.edu/
JPC Diagnoses:
1. Heart: Endocarditis, myocarditis, and valvulitis, chronic-active, suppurative, diffuse, with septic thrombus, granulation tissue, and numerous cocci.
2. Lung, pulmonary arteries: Arteritis, necrotizing, multifocal, severe, with septic thrombi and numerous cocci.
JPC Comment:
Group B Streptococci, also known as Streptococcus agalactiae, are opportunistic bacteria that are estimated to colonize the gastrointestinal and urogenital tracts of 1 in 4 humans.3 GBS are divided into 10 different serotypes based on the composition of their polysaccharide capsules, with different serotypes causing more or less virulent disease with implications for patient prognosis. Invasive GBS infection is causing growing levels of mortality and morbidity among neonates,
pregnant women, the elderly, and those with underlying health conditions. Invasive GBS infections have also been reported in primates, dogs, fish, and cattle, and rodents and primates have been used as experimental models of infection both neonatal and adult GBS infection.3
In laboratory rats, case reports of spontaneously occurring GBS infections often recapitulate late-onset GBS infections in humans, with infections characterized by myocarditis, metritis, meningitis, and septicemia.3 Comparative phylogenetic analyses from animals as diverse as tilapia, cattle, and humans show that GBS strains are extremely closely related, and thus could represent an anthropozoonotic risk. Whether such trans-species transmission actually occurs is an active area of research; in the meanwhile, personal protective equipment and training on preventive measures for laboratory animal handlers is recommended.3
Discussion of this case centered initially on cardiac anatomy, with conference participants divided on whether the cardiac thrombus was located in the right or the left side of the heart. Dr. Alves noted that sequelae of a left-sided thrombus would be widely dissementated thrombus formation, while right sided thrombi might be expected to propagage clots within the pulmonary vasculature, as seen in this case. Duly convinced that the thrombus likely began in the heart, partipants noted the multiple small caliber blood vessels in the myocardium, particularly at the apex of the heart, and agreed with the contributor that, while a surprising location, these likely represented granulation tissue subjacent to the completely denuded endocardium.
Gram staining highlighted the embolic nature of this disease process, with large colonies of gram-positive bacteria throughout the pulmonary vasculature. The moderator also noted the substantial interstitial pneumonia present in the lungs and characterized the infiltrate as primarily lymphoplasmacytic with fewer neutrophils using immunohistochemical staining, including a stain for myeloperoxidase (MPO), which helpfully highlighted neutrophils.
Dr. Alves discussed coagulation more broadly, revisiting Virchow’s triad of turbidity, endothelial damage, and hypercoagulability. In this case, the animal had recently given birth, and the hormonal changes associated with pregnancy and periparturition are known risk factors for hypercoagulability. The inflammatory conditions created by the massive bacterial infection could also cause vascular changes leading to slowing of blood flow and endothelial damage which could contribute to turbidity and exposure of tissue factor, all of which increase the risk of thrombus formation.
Conference participants preferred not to include the notable interstitial pneumonia in the morphologic diagnoses as it was likely secondary to the main process; however, the interstitial pneumonia was seen, acknowledged, and appreciated. Conference participants discussed the thrombus location within the heart at length, particularly whether it was located in the atrium or the ventricle. Participants noted the contributor’s location of right atrium, which is undoubtedly correct; however, based on the slide evaluated at conference, participants could not definitively localize the thrombus and hedged with a generally correct location of “heart.”
References:
1. Albers TM, Henderson KS, Mulder GB, Shek WR. Pathogen prevalence estimates and diagnostic methodology trends in laboratory mice and rats from 2003 to 2020. JAALAS. 2023;62(3):229-242.
2. Barthold SW, Griffey SM, Percy DH. Pathology of Laboratory Rodents and Rabbits. 4th ed. Blackwell Publishing; 2016.
3. Bodi Winn C, Bakthavatchalu V, Esmail MY, et al. Isolation and molecular characterization of group B Streptococcus from laboratory Long-Evans rats (Rattus norvegicus) with and without invasive group B streptococcal disease. J Med Microbiol. 2018;67(1):97-109.
4. Cooper TK, Meyerholz DK, Beck AP, et al. Research-relevant conditions and pathology of laboratory mice, rats, gerbils, guinea pigs, hamsters, naked mole rats, and rabbits. ILAR J. 2021;62(1-2):77-132.
5. Hansen AK, Nielsen DS, Krych L, Hansen CHF. Bacterial species to be considered in quality assurance of mice and rats. Lab Anim. 2019;53(3):281-291.
6. Mähler C, Berard M, Feinstein R, et al. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim. 2014; 48(3):178-192.
7. Otto GM. Chapter 4 - Biology and Diseases of Rats. In: Laboratory Animal Medicine. 3rd ed. Boston Academic Press; 2015.
8. Shuster KA, Hish GA, Selles LA, et al. Naturally occurring disseminated group B streptococcus infections in postnatal rats. Comp Med. 2013 Feb;63(1):55-61.