CASE IV: N2017-0533 (JPC 4117674).
Signalment:
Juvenile, undetermined sex, tentacled snake (Erpeton tentaculatum)
History:
Multifocal skin bumps were noticed on the skin of a juvenile tentacled snake, and two days later the snake was found dead.
Gross Pathology:
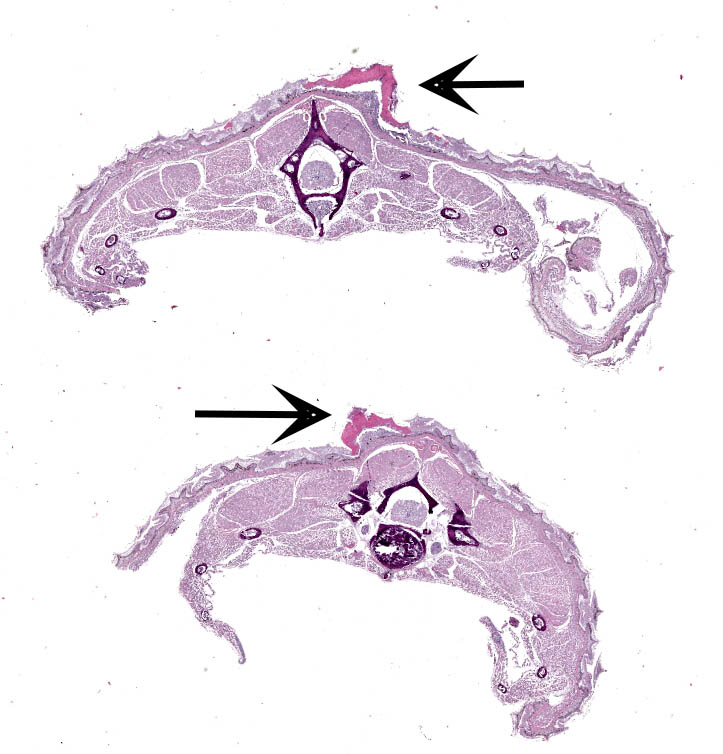
Along the skin, predominantly along the caudal half of the body, are dozens of multifocal, 0.1 cm to 0.2 cm, white to tan, soft to gritty, variably raised up to 0.1 cm, exophytic foci. On cut section these foci are limited to the scaled skin and do not involve subjacent tissue.
Laboratory Results:
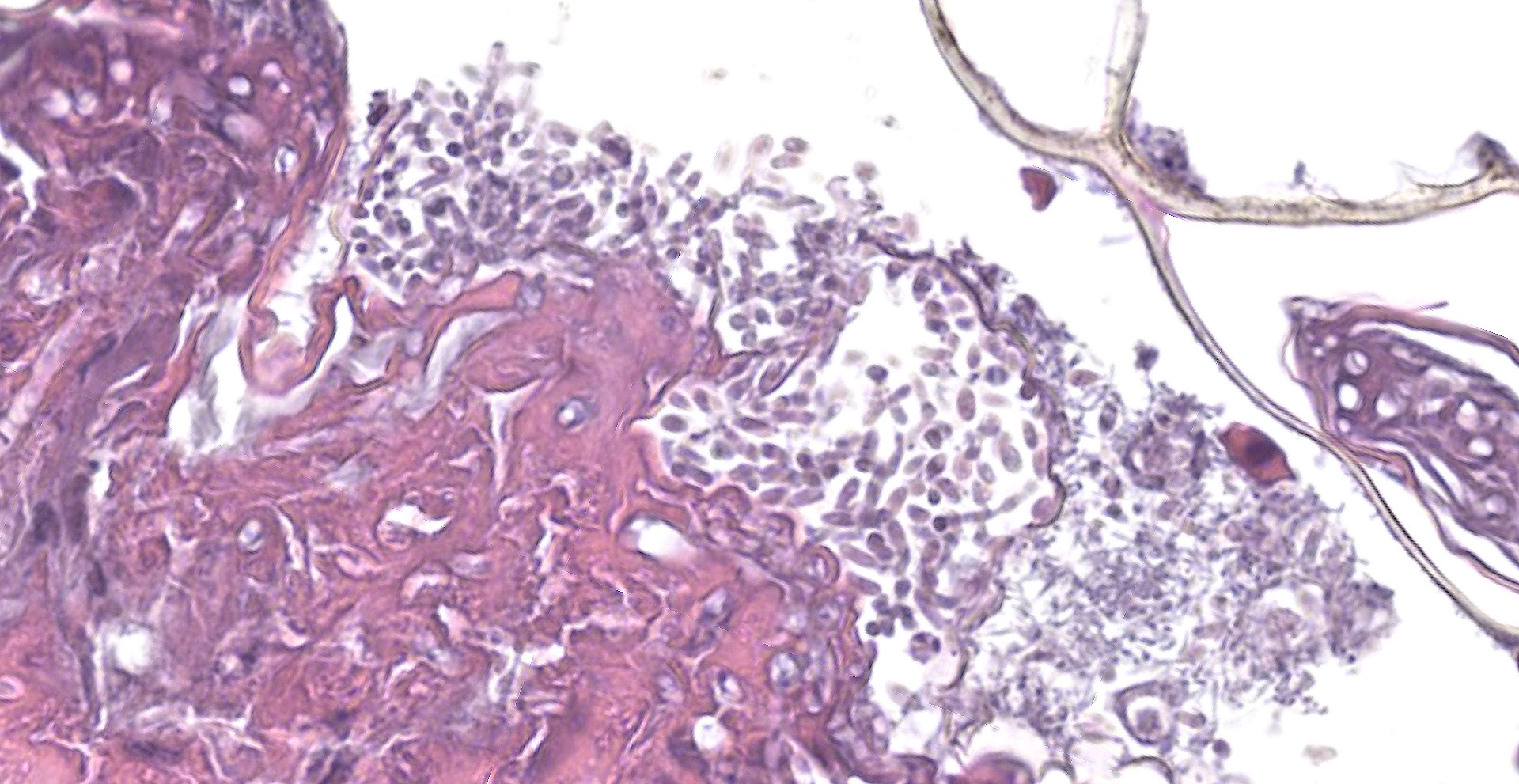
Cytology: A touch impression of a skin lesion stained with Diff-Quik shows small aggregates of amorphous purple-staining keratinaceous debris mixed with numerous oval to pyriform conidia, rare elongating hyphal forms, and moderate mixed, extracellular bacteria (predominately bacilli with few cocci).
A fresh-frozen sample of the skin lesions was PCR positive for fungus at the Zoological Medicine and Wildlife Disease Laboratory at the University of Florida, and direct sequencing identified Paranannizziopsis australasiensis.
Microscopic Description:
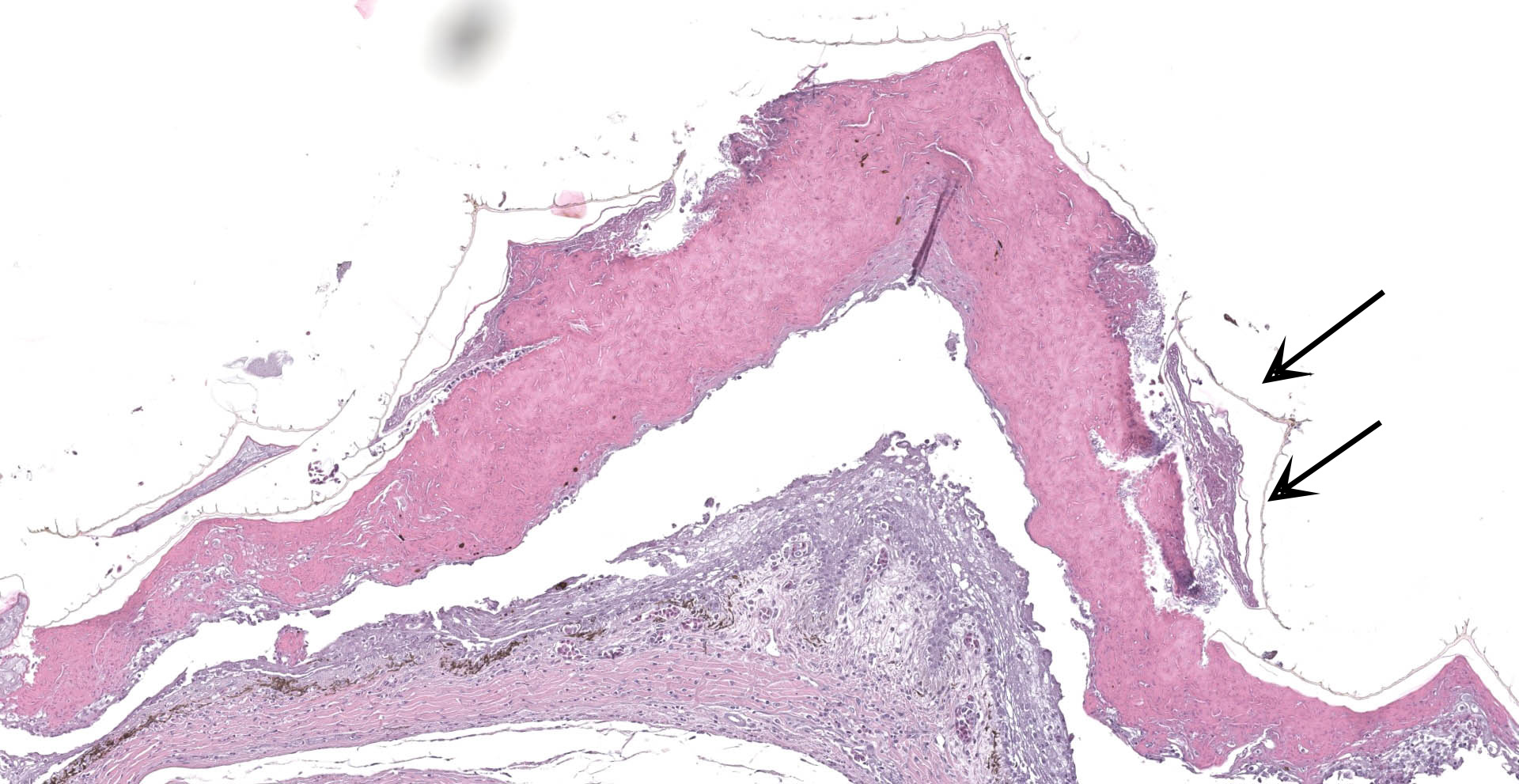
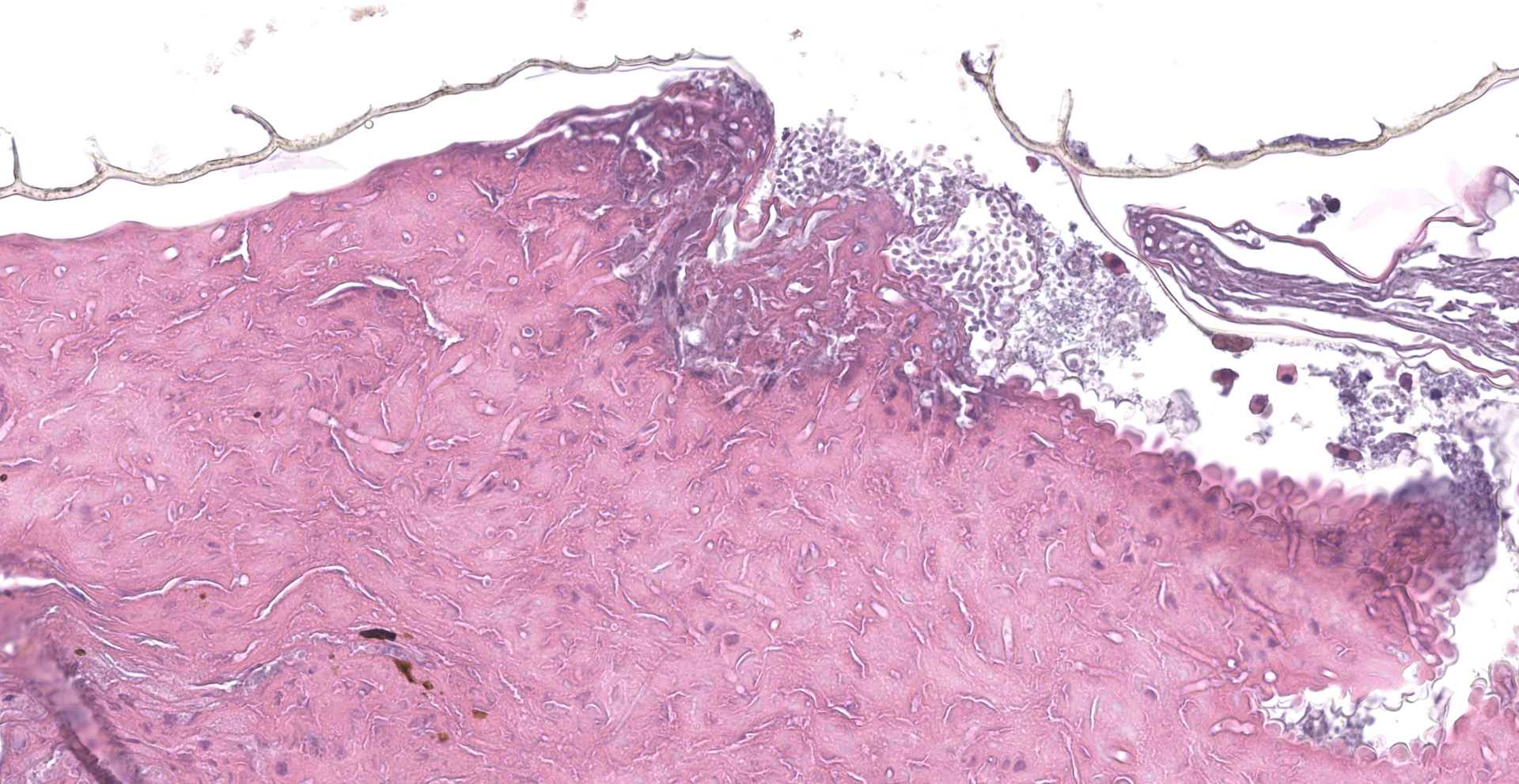
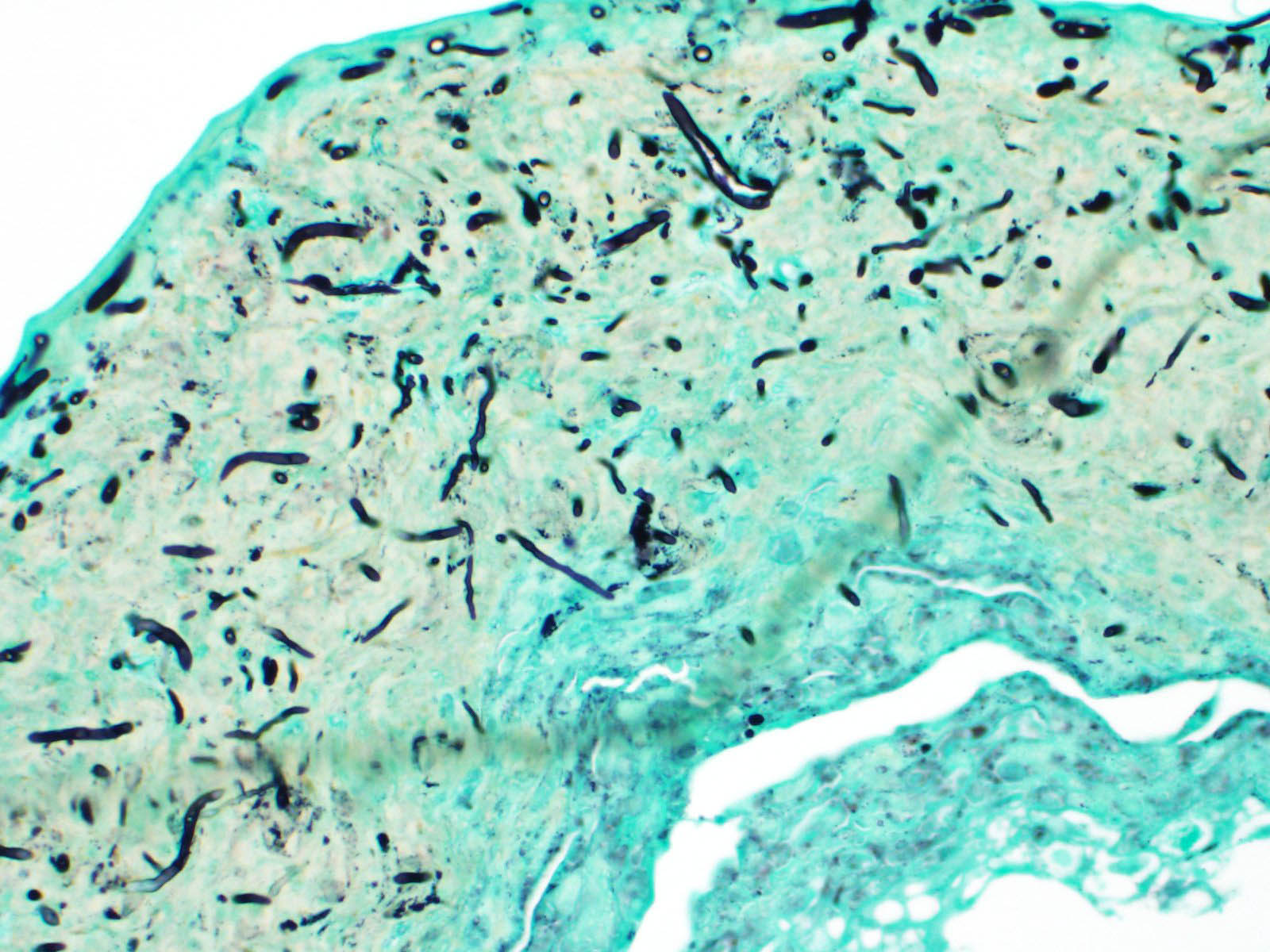
Scaled skin: Multifocally, there is mild to marked expansion and replacement of the epidermis by dense, brightly eosinophilic, degenerate heterophils, necrotic debris, degenerate keratin and variably discernable shrunken or vacuolated keratinocytes with pyknotic nuclei. Within the necroinflammatory crusts are moderate numbers of non?pigmented fungal hyphae, approximately 3-5 ?m wide, with parallel walls, regular septa, and frequent acute to right angle branching. Along the surface and often elevating the beta-keratin, are small to moderate numbers of oval-shaped conidia, approximately 2 x 4 ?m. Small to moderate numbers of mixed bacilli and cocci bacteria, and occasional foreign debris are also present along the epidermal surface. The adjacent epidermis is moderately hyperplastic with mild to moderate intracellular edema and low numbers of infiltrating heterophils. Frequently, there is segmental retention of the outer epithelial generation with a vacuolated lacunar tissue of outer generation, and separation from the beta-layer of inner generation (dysecdysis).Within the inner epidermis there is mild vacuolation of basal keratinocytes and occasional, mild, intercellular edema. The dermis is infiltrated by small to moderate numbers of heterophils, and small numbers of perivascular to interstitial lymphocytes and histiocytes.
Contributor's Morphologic Diagnoses:
Scaled skin: Epidermitis, heterophilic and necrotizing, multifocal, chronic, moderate with moderate numbers of intralesional fungal hyphae and conidia, moderate mixed superficial bacteria, moderate epidermal hyperplasia, mild lymphohistiocytic
dermatitis, and mild to moderate dysecdysis.
Contributor's Comment:
This case demonstrates a mycotic dermatitis (dermatomyosis) caused by Paranannizziopsis australasiensis. While previously known under the name Chrysosporium anamorph of Nannizziopsis vriesii (CANV), more recent phylogenetic studies have separated CANV into the genera Nannizziopsis, Ophidiomyces and Paranannizziopsis.6,8 Multiple different species of these genera are important causes of dermatitis in reptiles. Two of the more widely reported members of these genera include Nannizziopsis guarroi, the primary agent in "yellow fungus disease", a deep granulomatous dermatomycosis affecting bearded dragons, and Ophidiomyces ophiodiicola the causative agent of snake fungal disease (SFD). SFD is an important cause of morbidity and mortality in both captive and wild snakes, and is recognized as a major threat to wild snakes in the north central and northeastern United States.3,6
The fungus Paranannizziopsis australasiensis, has been recognized as a cause of dermatitis in reptiles (snakes, lizards and tuataras) in New Zealand and Australia; however, it has not been previously reported in North America.1,2,5 Other Paranannizziopsis spp. (P. californiensis, P. crustacea, and P. longispora) are reported to cause dermatitis in captive tentacled snakes in North America and are a significant infectious disease.6
All species in the Nannizziopsiaceae family produce single-celled aleurioconidia, and most species additionally produce arthrocondidia. The production of conidia differs between fungal species and between in vitro versus in vivo environments. Aleurioconidia can take multiple shapes (e.g., oval, pyriform, clavate), whereas arthroconidia are classically cylindrical and appear rectangular on side view.6,8 In culture, P. australasiensis is reported to produce pyriform to clavate aleurioconidia and occasional arthroconidia.8
Dermatomycosis in snakes can result in extensive disruption of the skin with subsequent secondary bacterial infections, dysecdysis, and osmotic imbalances. Aquatic snakes, such as tentacle snakes, may be particularly susceptible to osmotic imbalances associated with cutaneous lesions.1 Additionally, lesions on the head and around the mouth, which have been reported to be particularly severe with Ophidiomyces ophiodiicola, can result in facial deformity and emaciation.3,6
Contributing Institution:
JPC Diagnosis:
Scaled skin: Epidermitis, necrotizing, multi-focal, marked with numerous fungal hyphae and arthroconidia.
JPC Comment:
The contributor provides an excellent overview of three significant causes of mycotic dermatitis in reptiles formerly classified as Chrysosporium anamorph of Nannizziopsis vriesii (CANV). In addition to snakes, these etiologies have been reported in multiple species including crocodilians, lizards, and tuataras.9
Tentacled snakes (Erpeton tentaculatum) are an aquatic species commonly sought by zoological collections for exhibits due to their unique appearance and feeding behavior. A 2021 review4 of 125 pathology reports of tentacled snakes at multiple New York City zoos between 1966 and 2017 found over half the deaths in this species were attributed to infectious disease. Over half (n=40) of these deaths were due to fungal dermatitis, with the majority of cases of fungal dermatitis demonstrating histomorphology consistent with Paranannizziopsis spp. Fungal dermatitis was followed by bacterial infection as a common cause of death (n=21), with 7 of these cases attributed to Mycobacterium spp.4
In addition to the species previously listed by the contributor, an additional species (P. tardicrescens) has been identified following the submission of this case to the WSC in 2018. Before this discovery, North American cases were restricted to only captive tentacle snakes with identified species included P. crustacea, P. californiensis, P. longispora, and P. australasiensis. Although the newly identified P. tardicrescens was identified in two tentacle snakes, the pathogen was also identified in two additional snake species, a Wagler's viper (Tropidolamemus wagleri) and a rhinoceros snake (Rhynchophis boulengeri) that had been housed in the same room prior to developing lesions. Although the source and route of transmission with these cases is unknown, a misting system servicing terrestrial aquaria adjacent to tentacled snake enclosure may have inadvertently resulted in the pathogen's distribution via water droplets. Given this finding, it is suspected P. tardicrescens is capable of spreading via fomites, highlighting the need for basic but strict biosecurity measures such as cleaning and disinfecting tools, workspaces, scales, as well as wearing and changing gloves between enclosures and hand washing. In addition, the water in both tentacled snake enclosures was found to have a pH of >7, which may have predisposed these snakes to infection. Wild tentacle snakes are typically found in slightly acidic water which is ideally mimicked in captivity by maintaining a pH range of of 6.0-6.5.7
P. tardicrescens is unique from other Paranannizziopsis species in that it demonstrates a variable growth pattern when cultured at 35?C depending on the medium while other species now no growth or strong growth restriction. Additional distinguishing features of P. tardicrescens include the production of urease and lack of undulate hyphal branches. In contrast, both P. australasiensis and P. californiensis are weak urease producers and P. crustacea has undulant hyphal branches.7
Although Nannizziopsis, Ophidiomyces, and Paranannizziopsis have been found to infect multiple reptilian species, they have not been found to infect aquatic turtles (order Testudines). However, another notable species within fungal order Onygenales is Emydomyces testavorans. This species was recently identified following its isolation from ulcerative shell lesions of freshwater aquatic turtles, including a variety of zoological collections as well as wild western pond turtles (Actinemys marmorata) in Washington State. E. testavorans forms narrow, septate hyphae similar to Nannizziopsis, Ophidiomyces, and Paranannizziopsis, however, arthroconidia are not observed in sections of shell or tissue lesions. In addition, conidia formed in culture are smaller than those of the previously described fungi.9
The moderator noted tentacle snakes infected with Paranannizziopsis spp. most commonly develop facial lesions whereas the previously described Wagler's viper and rhinocerous snakes developed lesions on the lateral aspect of the body. In addition, the most common cause of death of tentacle snakes infected with this entity is drowning.
The subject of dysecdysis (abnormal or impaired shedding of skin) was a topic discussion amongst participants in while discussing this case. The prevailing opinion amongst the majority was dysecdysis could not be differentiated from ecdysis (the normal periodic process of sloughing and renewal of skin) due to lack of more definitive features such as inflammation associated with retained outer generational layers of skin.
References:
1. Bertelsen MF, Crawshaw GJ, Sigler L, Smith D. Fatal cutaneous mycosis in tentacled snakes (Erpeton tentaculatum) caused by the Chrysosporium anamorph of Nannizziopsis vriesii. J Zoo Wild Med. 2005. 36(1): 82-87.
2. Humphrey S, Alexander Sand Ha HJ. Detection of Parannannissiopsis australasiensis in tuatara (sphenodon punctatus) using fungal culture and a generic fungal PCR. NZ Vet J. 2016. 64(5): 298-300.
3. Lorch JM, Knowles S, Lankton JS, et al. Snake fungal disease: an emerging threat to wild snakes. Philos Trans R Soc Lond B Biol Sci. 2016;371(1709):20150457. doi:10.1098/rstb.2015.0457
4. Mack ZE, Boyer DM, Cardillo S, Conley KJ. A REVIEW OF MORTALITY IN TENTACLED SNAKES (ERPETON TENTACULATUM) IN A MULTI-INSTITUTION ZOOLOGICAL COLLECTION BETWEEN 1966 AND 2017. J Zoo Wildl Med. 2021;52(3):909-917.
5. Masters NJ et al. Dermatomycosis caused by Paranannizziopsis australasiensis in five tuatara (Sphenodon punctatus) and a coastal bearded dragon (Pogona barbata) in a zoological collection in New Zealand. NZ Vet J. 2016. 64(5): 301-307.
6. Pare JA and Sigler L. An overview of reptile fungal pathogens in genera nannizziopsis, paranannizziopsis, and ophidiomyces. J Herpatol Med Surg. 2016. 26 (1-2): 46-53.
7. Rainwater KL, Wiederhold NP, Sutton DA, et al. Novel Paranannizziopsis species in a Wagler's viper (Tropidolaemus wagleri), tentacled snakes (Erpeton tentaculatum), and a rhinoceros snake (Rhynchophis boulengeri) in a zoological collection. Med Mycol. 2019;57(7):825-832.
8. Sigler L, Hambleton S, Pare JA. Molecular characterization of reptile pathogens currently known as members of the Chrysosporium anamorph of Nannizziopsis vriesii complex and relationship with some human-associated isolates. J Clin Micro. 2013. 51 3338-3357.
9. Woodburn DB, Miller AN, Allender MC, Maddox CW, Terio KA. Emydomyces testavorans, a New Genus and Species of Onygenalean Fungus Isolated from Shell Lesions of Freshwater Aquatic Turtles. J Clin Microbiol. 2019;57(2):e00628-18.