Signalment:
Two and a half-year-old, male beagle, canineThe dog died with multiple bite wounds inflicted by other dogs kept in the same kennel
Gross Description:
At necropsy the animal displayed multifocal, severe epidermal ulcers and excoriations of the skin of the neck, thorax and both hind limbs associated with multifocal subcutaneous and intramuscular hemorrhages. The mandibular and retropharyngeal lymph nodes were enlarged and severely hyperemic. The endocardium had multiple petechial hemorrhages and the atrioventricular valves had mild nodular endocardiosis. The liver and lung were moderately congested. In addition, the lung had mild alveolar edema and emphysema.
Histopathologic Description:
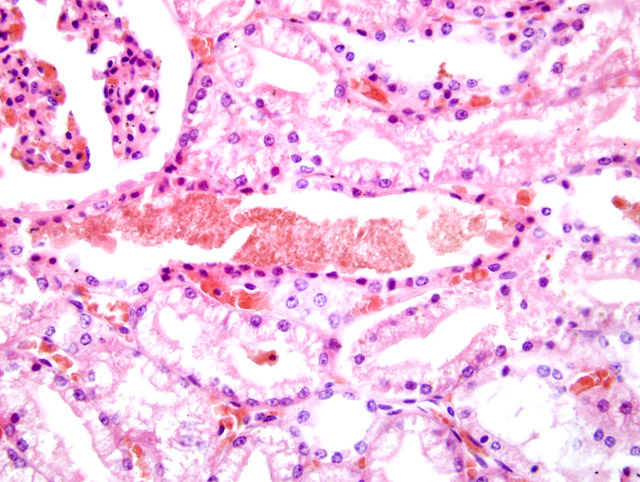
Within the kidney there was multifocal vacuolation, degeneration and necrosis with sloughing and loss of tubular epithelial cells. Epithelial degeneration and necrosis were frequently associated with small cytoplasmic granular deposition of a brown-greenish pigment. Tubuli were multifocally moderately dilated and contained hyalin or coarsely granular eosinophilic to brown-greenish casts
(Fig 2-1). The Bowman-�s capsule spaces contained abundant eosinophilic, proteinaceous material. Interstitial and glomerular blood vessels were moderately congested with multifocal prominent dilation of cortical veins.Â
A Turnbull blue stain identified iron in the tubular casts, the brush border and in cytoplasmic granular deposits of the tubular epithelium, consistent with chromoproteinuria.
Morphologic Diagnosis:
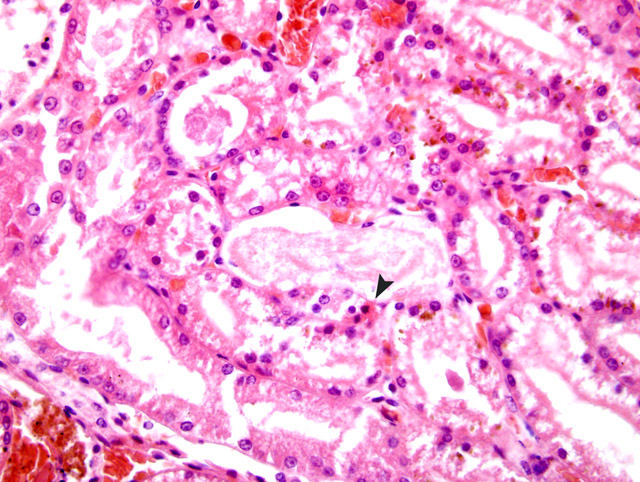
Kidney: Tubular degeneration and necrosis
(Fig. 2-2), acute, moderate, multifocal with cytoplasmic pigment deposition and intratubular chromoprotein casts.
Condition:
Traumatic rhabdomyolysis, myoglobinuric nephrosis
Contributor Comment:
The lesions are consistent with acute tubular necrosis following traumatic rhabdomyolysis and chromoproteinuria. Myoglobinuria as a consequence of elevated myoglobin serum concentration can be seen in metabolic dysfunction (e.g. equine exertional rhabdomyolysis, tying up), stress (e.g. capture myopathy) or severe direct trauma to muscles. In cases of traumatic injury, animals commonly also have renal hypoperfusion due to hypovolemic shock. The proposed mechanisms involved in myoglobinuria-induced renal injury include renal vasoconstriction, intraluminal cast formation and direct intra- and/or extracellular toxicity of myoglobin. It has been argued that renal vasoconstriction is due to extravasation of fluid in areas of damaged muscle tissue leading to intravascular volume depletion. Furthermore, activation of cytokine cascades and scavenging of nitric oxide as an important endogenous vasodilatator by heme protein contribute to renal hypoperfusion. In contrast to earlier views, it has been shown that the intraluminal cast formation during myoglobinuria enhances the dose dependent toxicity of heme by its accumulation and uptake and not by intratubular obstruction. Additionally, hypovolemia, renal vasoconstriction and loss of myoglobin solubility in acidic urine facilitate the formation of casts. The exact mechanisms of direct myoglobin toxicity are still under investigation. It has been hypothesized that by heme protein endocytosis, tubular plasma membranes become more vulnerable to the effects of phospholipase A2. Furthermore, iron dependent mechanisms of cellular damage including formation of free radicals, subsequent oxidative stress and lipid peroxidation have been proposed. Acute tubular necrosis is the most common reason for acute renal failure. Early degenerative lesions commonly seen with acute renal failure include loss of brush borders, flattening of the epithelium, detachment of cells, disruption of tubular basement membranes, formation of intratubular casts, and dilation of the lumens. These changes are observed predominantly in proximal tubules, but injury can also be demonstrated in the distal nephron and may progress to signs of necrosis like hypereosinophilia and loss of cellular detail. The distal nephrons seem to be secondarily damaged by obstruction with desquamated cells, cellular debris, hemoglobin, myoglobin, and other plasma proteins. Tubular regeneration, represented by flattened to elongated epithelial cells with hyperchromatic nuclei and mitosis can be seen after about three days. Within 2-3 weeks after toxin exposure, recovery of normal renal structure may be completed. Other conditions damaging renal tubular epithelium may result in morphologic changes similar to the lesions described here. However, the pigment deposition seen in this case is regarded as specific for hemoglobinuria, myoglobinuria or bilirubinuria. Other common nephrotoxins producing specific acute tubular necrosis in domestic animals include heavy metals (e.g. mercury, lead, arsenic), antibiotics, antifungal agents, anti-inflammatory drugs, and fungal, bacterial and plant toxins.Â
JPC Diagnosis:
Kidney: Degeneration and necrosis, tubular, acute, multifocal, moderate, with orange-red-brown casts, Beagle (
Canis familiaris), canine.
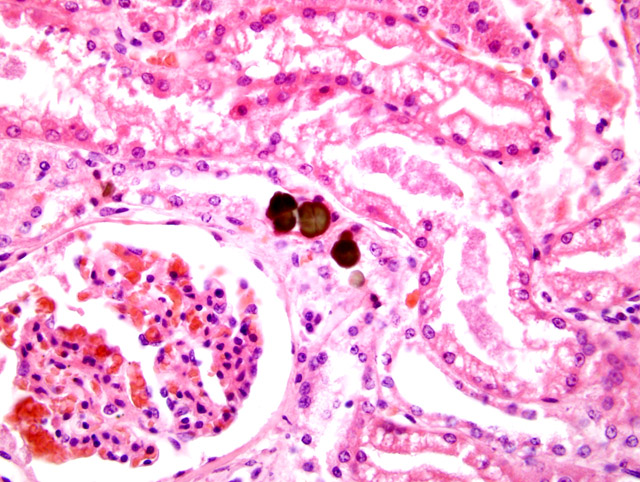
Kidney: Anisotropic green-brown crystals, intratubular, multifocal
(Fig. 2-3).
Conference Comment:
The contributor gives an excellent overview of myoglobinuric nephrosis. Hemoglobin and myoglobin are chromoproteins that have been associated with hemoglobinuric nephrosis or myoglobinuric nephrosis respectively. Hemoglobin is normally bound to the carrier protein haptoglobin, which is too large to be filtered by the glomerulus. Therefore, hemoglobin is not excreted in the urine unless supplies of the carrier molecule are depleted. Hemoglobin and myoglobin have little nephrotoxicity by themselves
4,6, but when associated with renal ischemia, acidic urine, and decreased glomerular filtration rate, they contribute to acute renal failure.
6
It is generally accepted that vasoconstriction, lipid peroxidation, and acidification of the urine all play roles in acute tubular necrosis. Cast formation is thought to result from decreased urine flow associated with a decreased GFR.
1,3 In vitro studies of myoglobin toxicity in Fischer 344 rats suggest primary mechanisms of damage result from diminished pyruvate-stimulated gluconeogenesis, decreased total glutathione levels and induction of lipid peroxidation.
5 The exact mechanisms for these actions and their effect in vivo are not fully known.Â
Hematuria, hemaglobinuria, and myoglobinuria will all generate a positive occult blood test. They can be differentiated by various diagnostic tests.
2 Centrifugation will cause sedementation of erythrocytes leaving a clear supernatant with hematuria. Red-brown urine that does not clear upon centrifugation may be either hemoglobinuria or myoglobinuria. These may be differentiated by adding saturated ammonium sulfate solution, which will precipitate hemoglobin. A clear supernatant following ammonium sulfate addition is indicative of hemoglobinuria, while a red-brown color indicates myoglobinuria.Â
Pigmentary changes in the kidney, extracted from Maxie et al.
4 and Newman et al.
6
| Condition | Pigment | Gross lesion | Histologic lesion |
| Hemoglobinuric nephrosis (acute hemolytic crisis) | Hemoglobin | Dark red-brown to blue-black with radial streaks | Fine red granular speckling within epithelial cells or granular casts |
| Myoglobinuric nephrosis (acute rhabdomyolysis) | Myoglobin | Dark red-brown to blue-black with radial streaks | Fine red granular speckling within epithelial cells or granular casts |
| Hemosiderosis (chronic hemolytic anemia) | Hemosiderin | Brown discoloration of cortex | Pigment within the epithelial cells of proximal tubules |
| Cloisonn+�-� kidney (non-clinical condition) | Ferritin and hemosiderin | Brown to black renal cortices | Brown pigmentation of basement membrane, convoluted portions of proximal tubules |
| Lipofuscinosis | Brown iron-free pigments | Radial dark lines on the cut surface of cortex, sparing the medulla | Fine brown granules in epithelial cells of convoluted tubules |
The green-brown intratubular crystals were identified by scanning electron microscopy with energy dispersive x-ray analysis (SEM-EDXA) and infrared spectroscopy (IR) as consistent with calcium oxalate monohydrate. The calcium oxalate crystals in this case are unusual in appearance because of the green-brown color in H&E. The crystals stained positive for Von Kossa and negative for Alizarin red. It is possible that protein and iron deposition within the crystals could account for their abnormal appearance. We would like to thank the AFIP Department of Environmental and Toxicologic Pathology for their assistance in evaluating this case.
References:
1. Efstratiadis G, Voulgaridou A, Nikiforou D, Kyventidis A, Kourkouni E, Vergoulas G: Rhabdomyolysis updated. Hippokratia 11:129-137, 2007
2. Gregory CR: Urinary system. In: Duncan & Prasse's Veterinary Laboratory Medicine Clinical Pathology, eds. Latimer KS, Mahaffey EA, Prasse KW, 4th ed., pp. 240-241. Iowa State Press, Ames, Iowa, 2003
3. Holt S, Moore K: Pathogenesis of renal failure in rhabdomyolysis: the role of myoglobin. Exp Nephrol 8:72-76, 2000
4. Maxie MG, Newman SJ: The urinary system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, pp. 475476. Elsevier Limited, St. Louis, MO, 2007
5. Minigh JL, Valentovic MA: Characterization of myoglobin toxicity in renal cortical slices form Fischer 344 rats. Toxicol 187:77-87, 2003
6. Newman SJ, Confer AW, Panciera RJ: Urinary system. In: Pathologic Basis of Disease, McGavin MD and Zachery JF, 4th ed., pp. 644-658. Mosby Elsevier, St. Louis, Missouri, 2007
7. Zager RA: Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int 49:314-326, 1996