Wednesday Slide Conference 2023-2024, Conference 3 Case 3
Signalment:
6-month-old, intact male Göttingen minipig (Sus scrofa)
History:
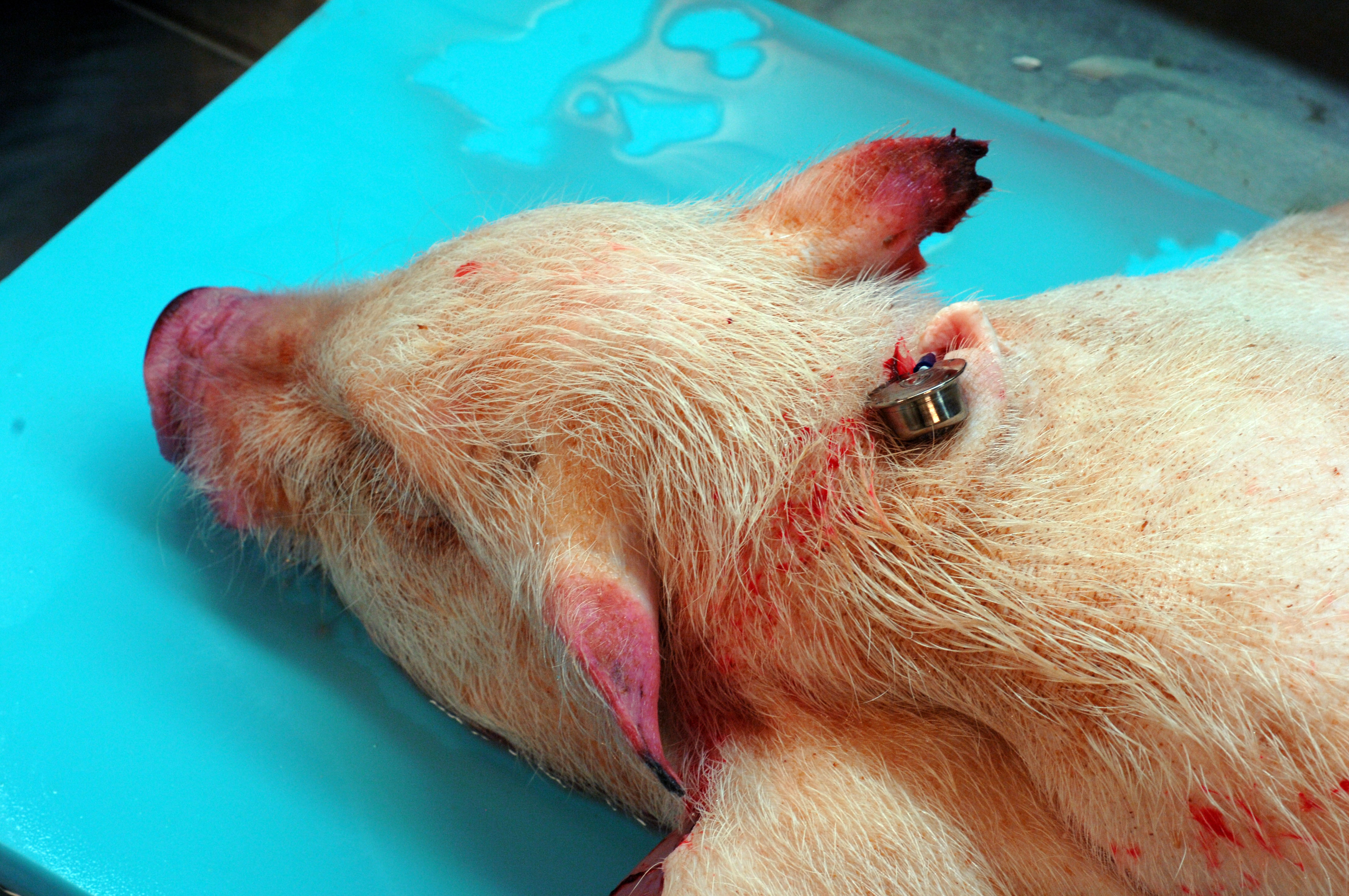
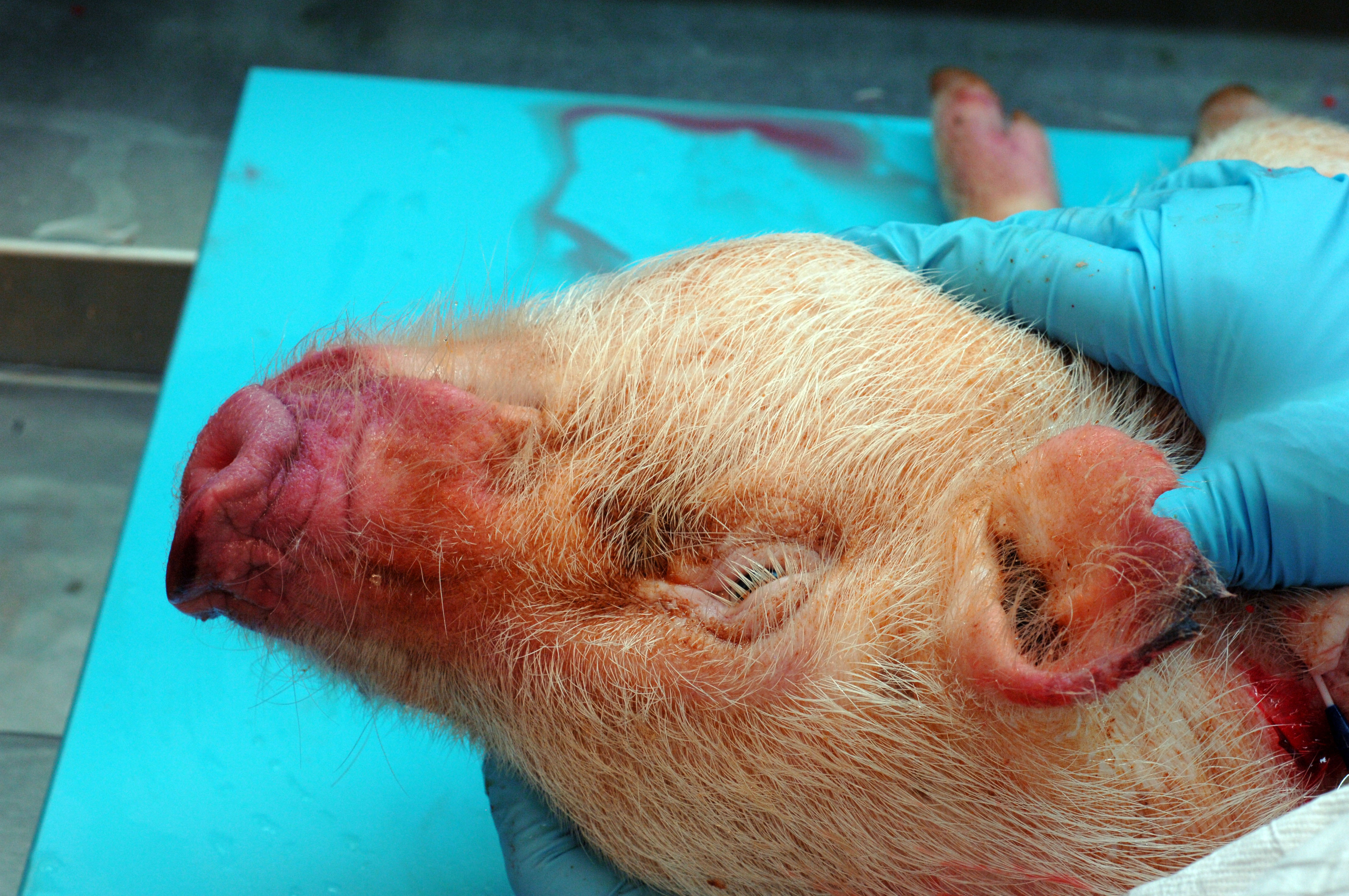
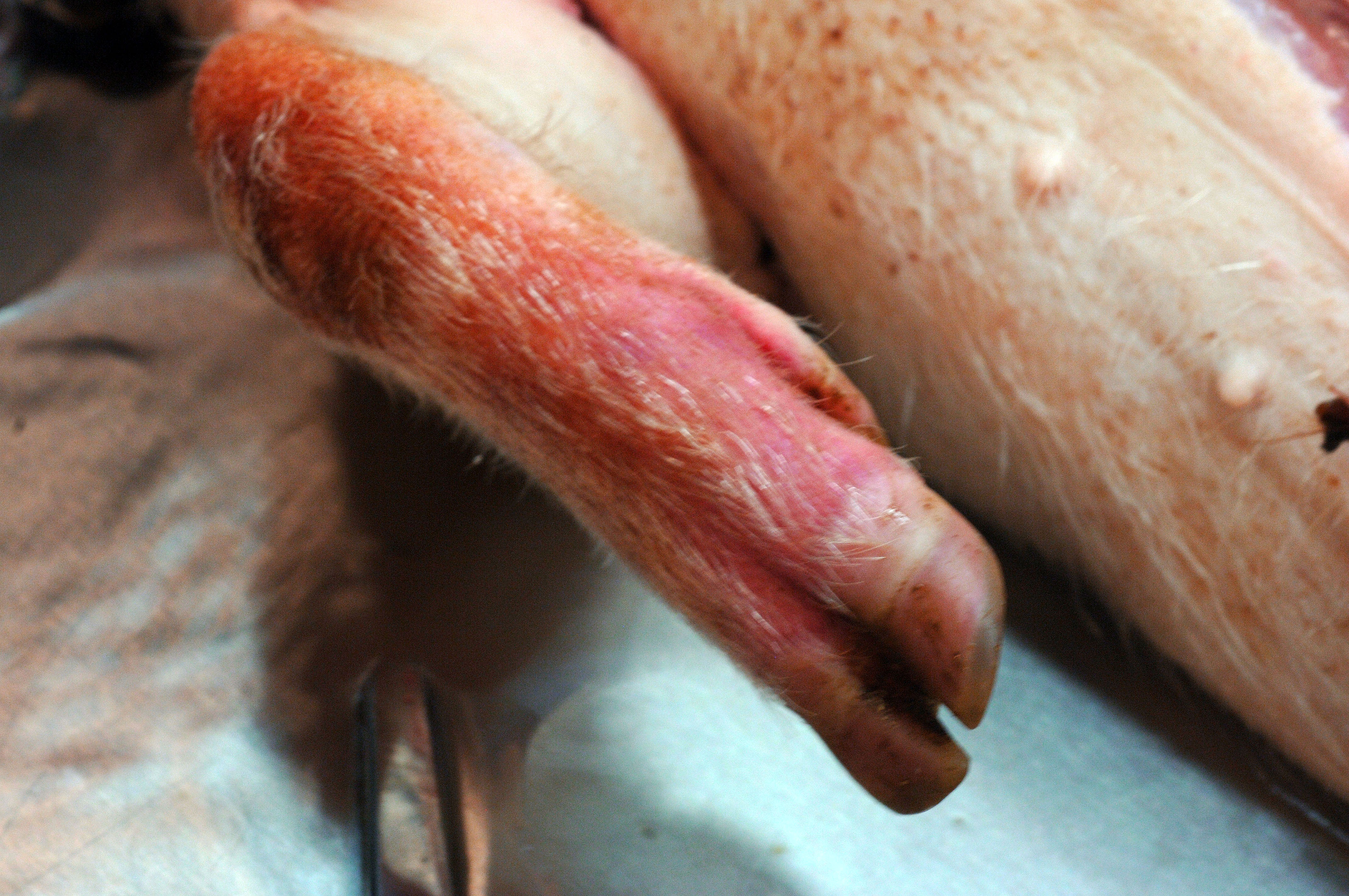
A 6-month-old, intact male, single-housed, 12 kg, Göttingen minipig presented with bilateral ear erythema at the apical margins 4 hours post repetitive auricular venipuncture. The pig had open cage bar communication with conspecifics. The pig had a jugular venous access port (VAP) and telemetry implanted 2 months prior to presentation. The pig was enrolled in a pharmacokinetics study 1 month prior and received 2 intramuscular doses of scopolamine. On exam, the pig was bright, alert, and responsive (BAR) with 10 needle punctures per ear and extensive bruising. At the 24 hour recheck, the pig was BAR but the erythema expanded the entire pinnae with ulceration, crusting, epidermal thickening, and apical margin necrosis. Aerobic culture revealed Staphylococcus aureus and povidone-iodine scrub with silver sulfadiazine therapy was initiated. Primary differentials included research/conspecific-induced trauma, bacterial, and/or viral systemic disease. At the 48 hour recheck, the pig presented with depression, inappetance, dyspnea, cold extremities, petechiation on snout, lips, rectum, and prepuce, and red-purple discoloration on all limbs distal to hock/elbow. Blood was taken for clinical pathology and was still watery 20 minutes later. The pig was euthanized due to poor study candidacy and disease severity.
Gross Pathology:
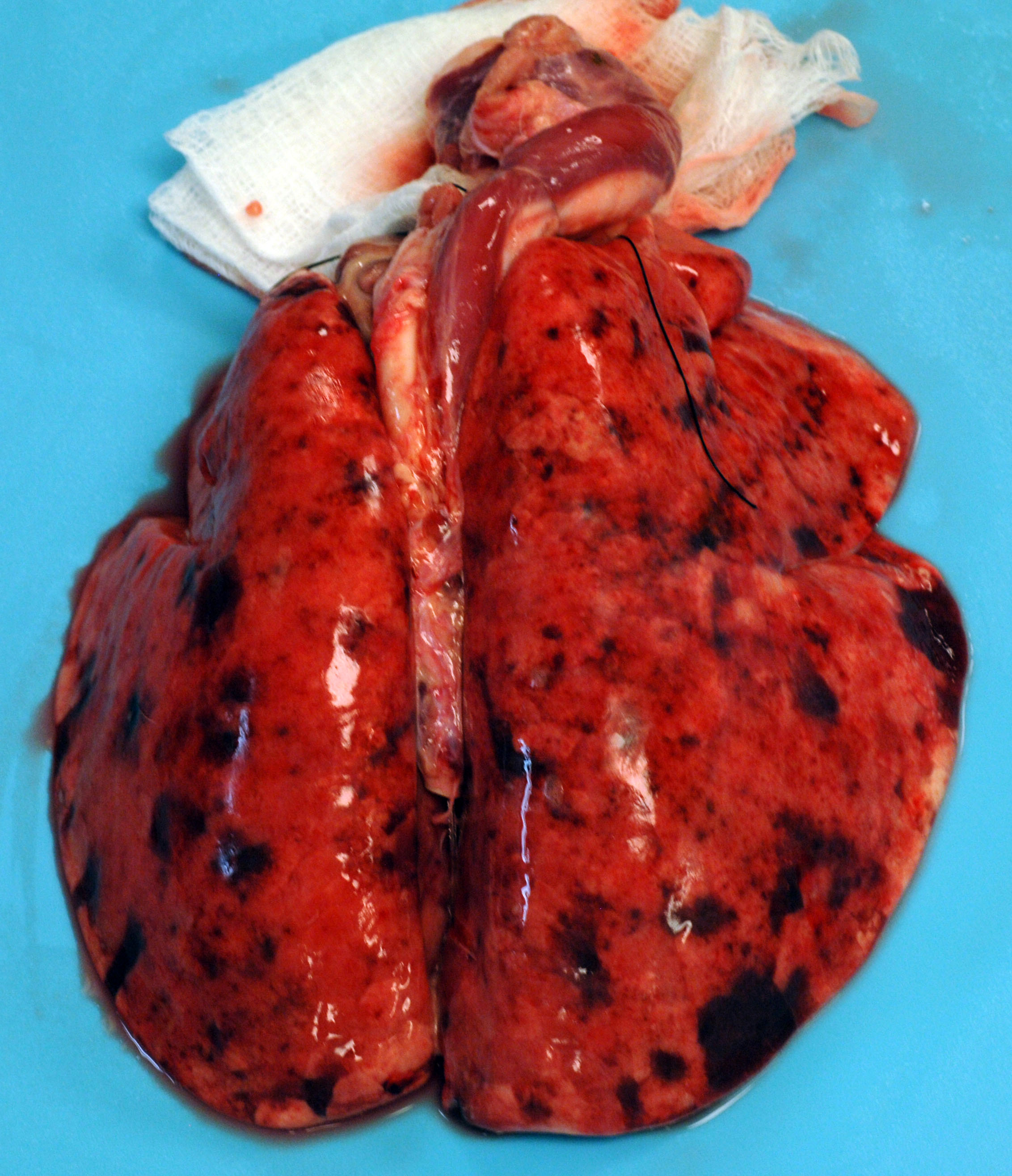
This 12 kg, male, Göttingen minipig is in lean body condition with adequate subcutaneous and visceral fat, 2.5/5 body condition score, and well-hydrated. There are multifocally extensive petechiae, purpura, and ecchymoses on the nose, ears, lips, lower limbs, ventrum, prepuce, anus, and tail. Bilaterally, the ear tips are dark & necrotic. The right pinna has a hole in the middle from an ear tag. There is a subcutaneous VAP port on the right lateral neck and a subcutaneous telemetry device on the right side of the ventral neck. Both have no significant gross lesions. The gums and conjunctiva are pale and there are multifocal petechiae on the palate. There is abundant hemorrhagic foam in the trachea. The lungs are noncollapsed and extensively dark with multifocal petechiae and purpura. There
are multifocal petechiae on the heart ventricles and there is a small amount of serosanguinous fluid in the pericardial sac. The liver, spleen, and kidneys are dark and enlarged (post-euthanasia congestion). The liver, kidneys, and intestinal serosa have multifocally extensive petechiae. The gallbladder contains a small amount of hemorrhagic bile. The stomach contains a moderate amount of normal ingesta and gas and there are multifocal petechiae and erosions/ulcers on the fundic mucosa. The small intestine contains a moderate amount of bile-stained digesta and gas. The cecum contains a moderate amount of dark hemorrhagic contents. The colon contains a small amount of normal feces. The urinary bladder is empty. No significant gross lesions were observed in any other organs.
Laboratory Results:
Clinical pathology revealed a regenerative anemia, low hemoglobin, marked thrombocytopenia, and elevated ALT, AST, GGT, and albumin. A differential count supported thrombocytopenia with schistocytosis, nucleated RBCs, acanthocytes, and target cells.
Microscopic Description:
Ear pinna: There is focally extensive full-thickness coagulative necrosis affecting the distal end. Multifocally there are vascular thrombi present and various arteriosclerotic changes to the arterioles and small muscular arteries (endothelial cell hypertrophy, myointimal proliferation, smooth muscle cell vacuolation, medial thickening, concentric laminar wall thickening (?onion-skinning?), luminal stenosis, and/or periarteritis). Inflammation is scattered and primarily composed of macrophages, lymphocytes, eosinophils, and neutrophils. There is also multifocal areas of periarticular hemorrhage and occasional hemosiderin-laden macrophages.
Changes in other organs (not submitted):
Heart: Multifocal severe myocardial fiber degeneration, necrosis, and loss with hemorrhage, vascular thrombi, and various arteriosclerotic changes to the arterioles and small muscular arteries.
Kidney, bilateral: Diffuse severe membranoproliferative glomerulonephritis with tubular degeneration, necrosis, and proteinosis, hemorrhage, vascular thrombi, and various arteriosclerotic changes to the arterioles and small muscular arteries.
Lung: Severe multifocally extensive intraairway and interstitial hemorrhage, fibrin, and edema with vascular thrombi and various arteriosclerotic changes to the arterioles and small muscular arteries.
Liver: Moderate to severe multifocal centrilobular to midzonal hepatocellular degeneration and necrosis with hemorrhage and vascular thrombi.
Stomach, fundus: Severe focally extensive mucosal necrosis with submucosal inflammation, edema, hemorrhage, vascular thrombi, and various arteriosclerotic changes to the arterioles and small muscular arteries.
Pancreas: Multifocal moderate acinar degeneration and necrosis with hemorrhage, vascular thrombi, and various arteriosclerotic changes to the arterioles and small muscular arteries.
Diaphragm: Multifocal moderate myofiber degeneration and necrosis with hemorrhage, vascular thrombi, and various arteriosclerotic changes to the arterioles and small muscular arteries.
Spleen: Diffuse moderate extramedullary hematopoiesis (EMH) with vascular thrombi and various arteriosclerotic changes to the arterioles and small muscular arteries.
Bone marrow: Diffuse moderate megakaryocytic hyperplasia.
Adrenal gland, bilateral: Multifocal moderate hemorrhage.
Gallbladder: Multifocal moderate submucosal hemorrhage and edema.
Ileum: Diffuse moderate GALT lymphocytolysis with vascular thrombi and various arteriosclerotic changes to the arterioles and small muscular arteries.
Duodenum: Multifocal vascular thrombi and various arteriosclerotic changes to the arterioles and small muscular arteries.
|
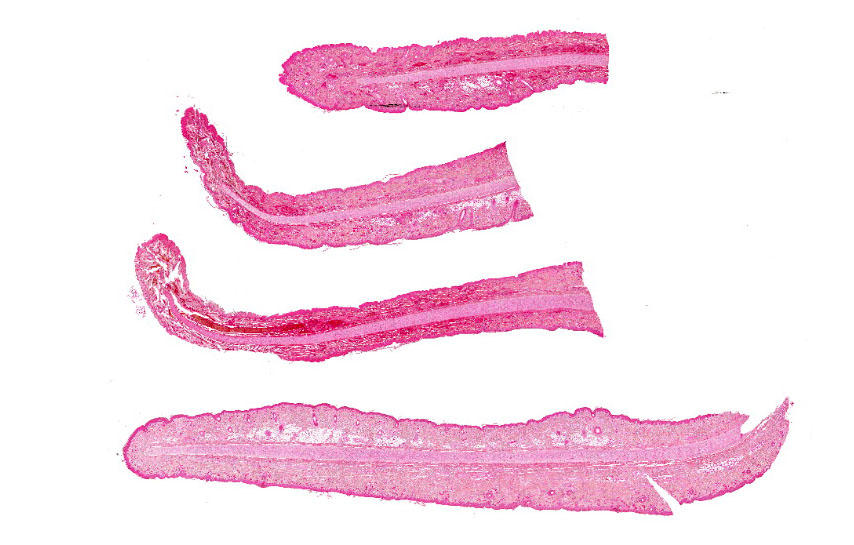
Figure 3-5. Ear pinnae, Göttingen minipig. Four sections of ear tip are submitted for examination. (HE 6X)
|
|
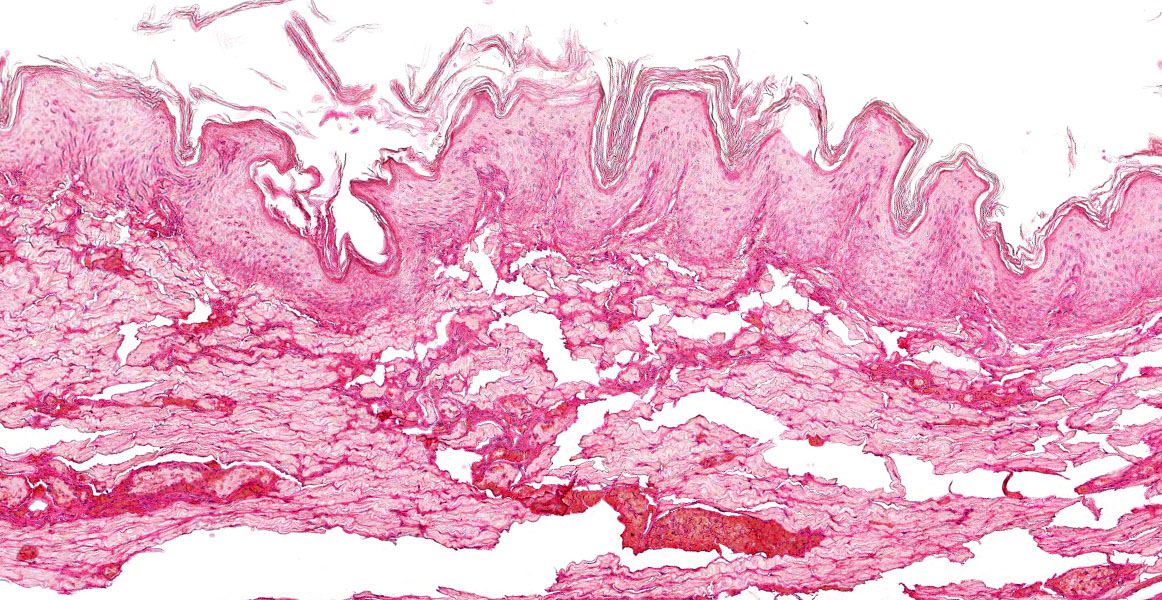
Figure 3-6. Ear pinna, Göttingen minipig. There is diffuse coagulative necrosis (infarction) with lack of stain affinitiy of the ear tip. (HE, 123X)
|
Lymph node, various: Multifocal moderate draining hemorrhage and lymphocytolysis with histiocytosis, erythrophagocytosis, hemosiderin-laden macrophages, vascular thrombi, and various arteriosclerotic changes to the arterioles and small muscular arteries.
Contributor?s Morphologic Diagnosis:
Ear pinna: Necrosis, full-thickness, focally extensive, severe, with vascular thrombi and various arteriosclerotic changes to the arterioles and small muscular arteries.
Contributor?s Comment:
Severe thrombocytopenia and anemia are consistent clinical findings with thrombocytopenia purpura syndrome of Göttingen minipigs, along with extensive hemorrhages in subcutaneous tissues and various other organs.1,6 This syndrome is a spontaneous degenerative vasculopathy that rarely occurs in European and North American herds of Göttingen minipigs.1,6 It primarily affects sexually mature males and females but has been seen in animals as young as 7 weeks of age.6 Histologically, there are distinctive features of arteriosclerosis in various tissues, primarily in small- to medium-sized arteries and arterioles. These features include endothelial cell hypertrophy, myointimal proliferation, smooth muscle cell vacuolation, medial thickening typically by myxoid deposits, concentric laminar wall thickening (?onion-skinning?), luminal stenosis, and/or periarteritis.6-8 Unlike spontaneous atherosclerosis of aged swine, lipid accumulation is not a feature.6 Membranoproliferative glomerulonephritis and myocardial infarcts also feature prominently in this disease.6,7 The pathogenesis is unknown at this time but it is proposed to be due to a type III hypersensitivity causing the widespread degenerative vasculopathy.1,6,7
Other rule-outs for cutaneous hemorrhages in pigs, although less likely in research bred animals, include: (1) neonatal thrombocytopenic purpura ? hypersensitivity due to incompatible colostral antibodies; (2) vitamin K deficiency/anticoagulant rodenticide poisoning; and (3) viral infections, such as porcine circovirus, classical swine fever, and African swine fever.
Contributing Institution:
Comparative Pathology Department, Research Support Division
USAMRICD
8350 Ricketts Point Rd.
Aberdeen Proving Ground, MD 21010-5400
https://usamricd.amedd.army.mil/
|
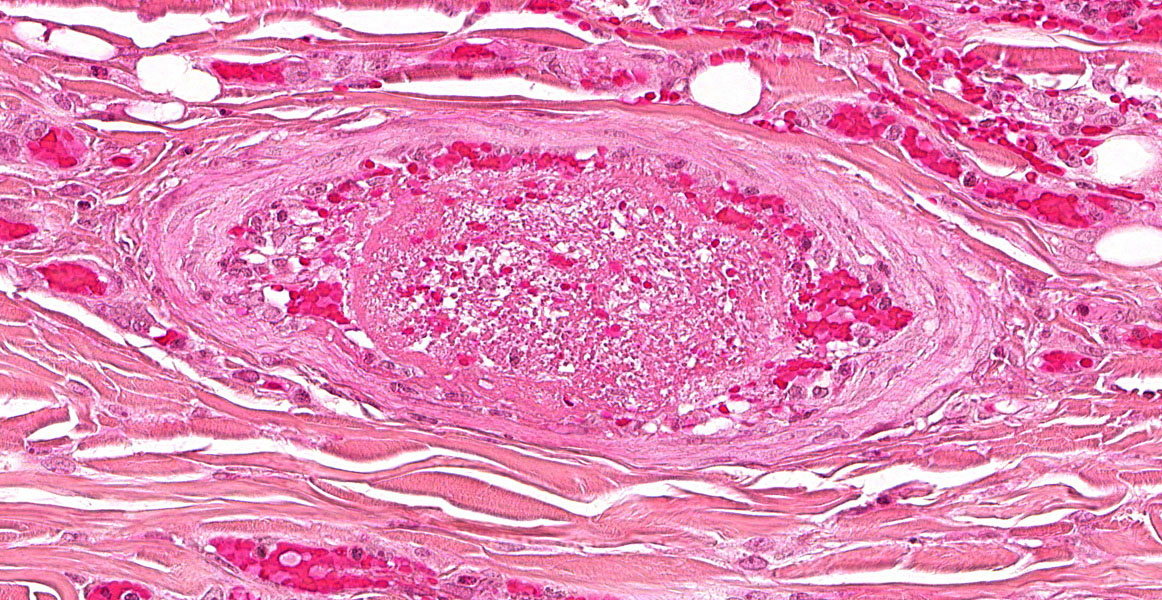
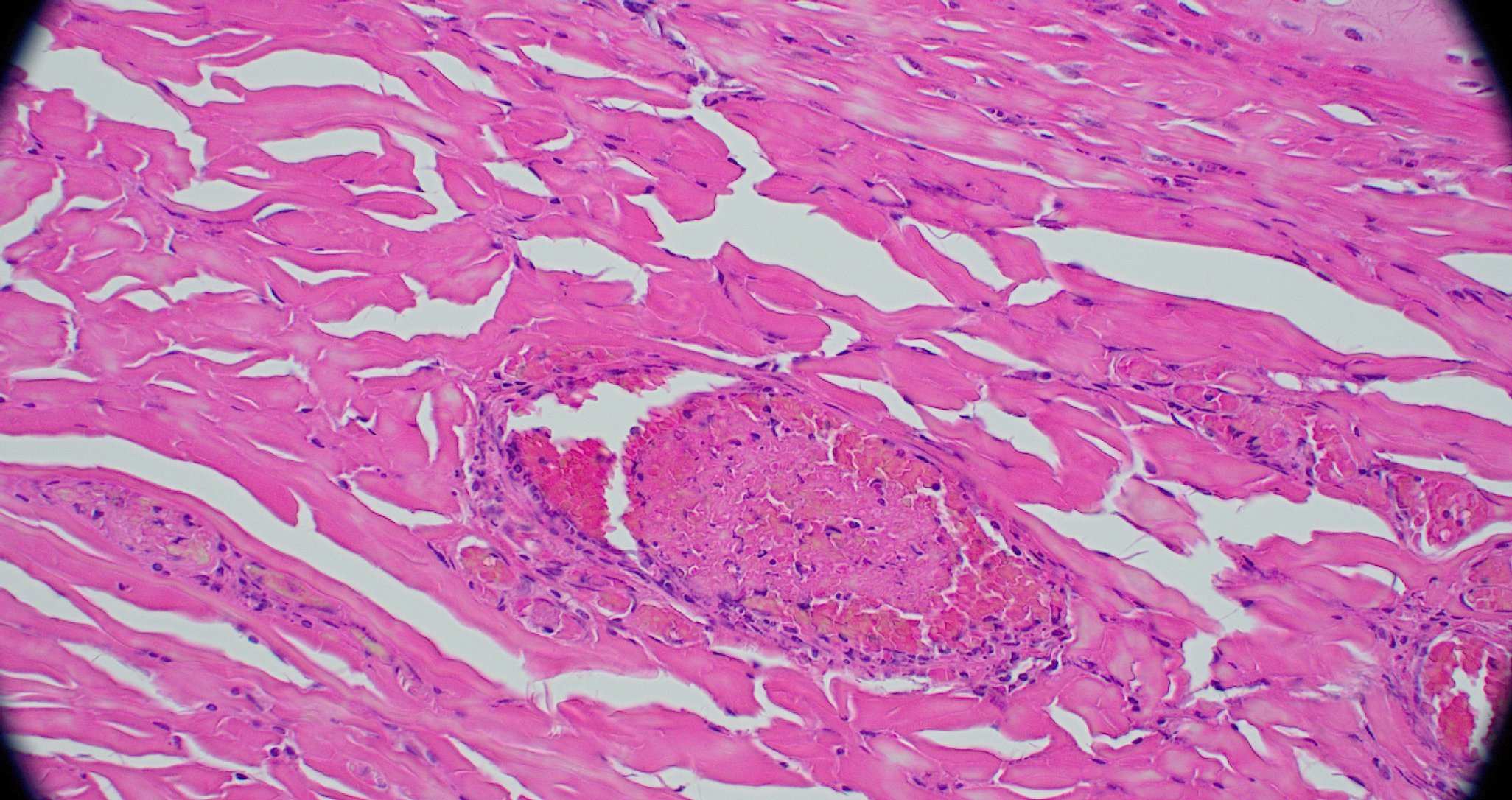
Figure 3-7. Ear pinna, Göttingen minipig. Dermal arterioles demonstrate mural thickening as a result of intimal hyperplasia and adventitial fibrosis and often contain non-occlusive fibrin thrombi. (HE, 387X)
|
|
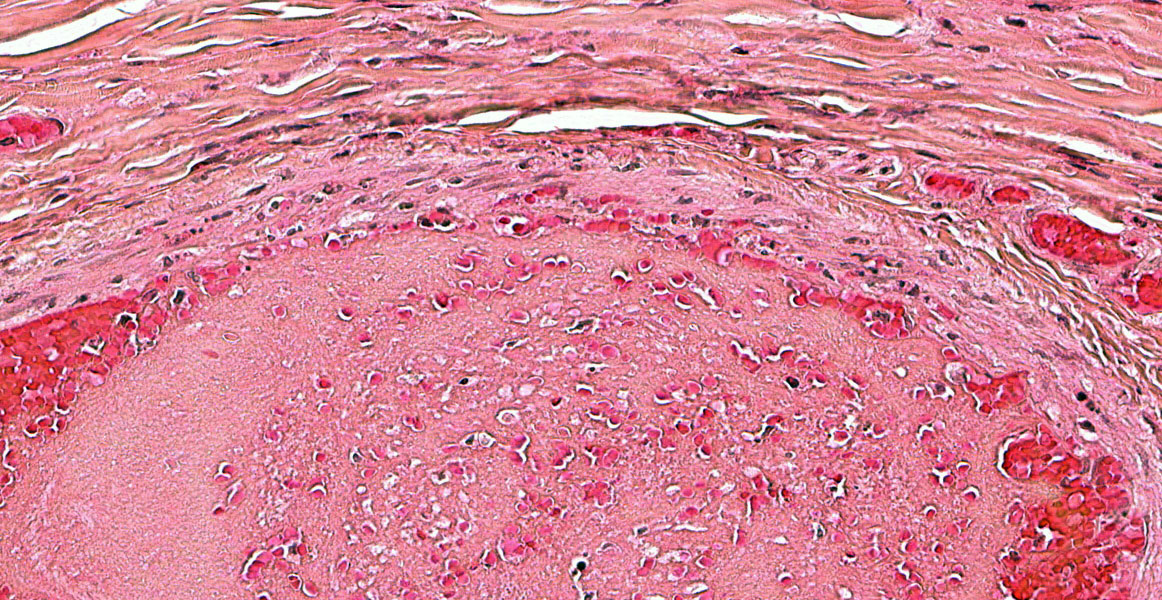
Figure 3-8. Ear pinna, Göttingen minipig. Occasionally, arteriolar walls contain exuded protein, necrotic smooth muscle, mural and adventitial necrotic neutrophils, and cellular debris. (HE, 508X)
|
JPC Diagnosis:
Haired skin, ear tip: Arteriolosclerosis, multifocal, moderate, with thrombosis, occasional arteritis and periarteritis, and focally extensive cutaneous infarction.
JPC Comment:
The syndrome nicely described by the contributor and illustrated by this case is a rare syndrome with an uncertain pathogenesis that is specific to Göttingen minipigs. As the contributor notes, one of the major differential diagnoses for this condition in swine is neonatal thrombocytopenia purpura, an immune-mediated condition in suckling pigs that has been described in veterinary literature for 50 years.5 The case presentation is similar to the syndrome in Göttingen minipigs, but with a much younger age of onset and a different pathogenesis.
In neonatal thrombocytopenic purpura, suckling pigs typically become symptomatic around 3 days of age, recover, and then experience a re-emergence of symptoms around day 14 which quickly leads to death.5 Piglets are pale and inactive and post-mortem exam reveals severe hemorrhage and edema in the lungs, heart, kidneys, subcutis, joints, gastrointestinal and respiratory tracts, and skeletal muscle. Histologic findings include hemorrhages in various tissues and depletion of megakaryocytes in the bone marrow and spleen.5
Thrombocytopenia develops due to isoimmune antibodies developed by the sow during gestation against boar thrombocyte antigens present in the piglets? blood. Piglets ingest these isoantibodies with colostrum which render existing platelets useless and have a cytotoxic effect on bone marrow megakaryocytes.5 Matings between Landrace and Large White pigs increase the risk that the resulting piglets will develop neonatal thrombocytopenia purpura.
Due to the need for prior sensitization, neonatal thrombocytopenia purpura occurs only on subsequent matings of a sow to the same boar. Because of this, the disease fell out of fashion with the advent of modern artificial insemination and the concomitant reduction in multiple identical matings.5 However, in a case of good intentions gone awry, the disease is being seen more frequently now that animal welfare preferences have catalyzed changes in livestock rearing practices. Of particular salience here is the use of natural mating as part of a larger push to allow animals to exhibit natural behaviors in all aspects of their lives.2 A recent outbreak occurred on a Swiss organic farm that practiced natural mating of pigs using one of only three boars kept on the property, increasing the chances of repeat matings.5 Thirteen piglets from two separate litters died of neonatal thrombocytopenia purpura before management practices, including exchanging boars periodically, documenting and managing matings, and switching all boars to the Duroc breed, were implemented.
The syndrome is similar to neonatal isoerythrolysis in foals in which anemia and subsequent organ failure result when foals inherit a blood type from the stallion against which the mare has previously developed antibodies. Similar to the syndrome described above, foals ingest the isoantibodies with colostrum which end up in the bloodstream and attach to the foal?s erythrocytes, causing their immune destruction.
Conference discussion centered on the pathogenesis of thrombocytopenia purpura of the Göttingen minipig. As the contributor notes, the current literature suggests that the degenerative vasculopathy characteristic of this disease is caused by a type III hypersensitivity reaction; however, causality has not been established and the moderator remains skeptical that the condition is solely immune-mediated. This case also provided a second opportunity to discuss the importance of a conscientious examination of vasculature, which here was multifocally thrombosed due presumably to intimal damage and the resulting exposure of collagen and tissue factor. The widespread thrombosis described by the contributor in various tissues likely resulted in a consumptive coagulopathy that explains the systemic, widespread hemorrhage seen in this animal. Due again to the centrality of the vasculature to the histologic presentation, the JPC morphologic diagnosis once again leads with the vascular changes.
References:
- Carrasco L, Madsen W, Salguero F, Núñez, Sánchez-Cordón P, Bollen P. Immune complex-associated thrombocytopenic purpura syndrome in sexually mature Göttingen minipigs. J Comp Path. 2003;128:25-32.
- Dawkins MS. Natural behavior is not enough: farm animal welfare needs modern answers to Tinberg?s four questions. Animals (Basel). 2023;13(6):988.
- Grand N. Diseases of minipigs. In: McAnulty P, ed. The Minipig in Biomedical Research. 1st ed. CRC Press; 2012.
- Helke K, Nelson K, Sargeant A, et al. Background pathological changes in minipigs: a comparison of the incidence and nature among different breeds and populations of minipigs. Tox Path. 2015; 44(3):325-337.
- Joller S, Hafliger IM, Drogemuller C, Richard OK, Grahofer A. Thrombocytopenic purpura on an organic farm with pen mating: a case report on the re-emergence of an old disease. Porc Health Manag. 2020;6(18).
- Maratea K, Snyder P, Stevenson G. Vascular lesions in nine Göttingen minipigs with thrombocytopenic purpura syndrome. Vet Pathol. 2006;43:447-454.
- McInnes E, McKeag S. A brief review of infrequent spontaneous findings, peculiar anatomical microscopic features, and potential artifacts in Göttingen minipigs. Tox Path. 2016;44(3):338-345.
- Swindle M, Makin A, Herron A, Clubb Jr F, Frazier K. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012;49(2):344-356.