Signalment:
8-month-old female domestic
shorthair cat (
Felis catus).Patient presented for lethargy,
depression, and inappetence. Rectal
temperature was 105.1F. Initial treatment
consisted of antibiotics, anti-inflammatory
drugs, and subcutaneous fluids. Five days
later, icterus with hypoglycemia, hypo-
albuminemia, and leucopenia were noted.
Rectal temperature was 103F. The patient
died the following day.
Gross Description:
The spleen was enlarged
with a roughened serosal surface. Multiple
white pinpoint foci were observed on the
serosal surface. The cut surface bulged and
had a granular appearance. No other gross lesions were indicated by the submitting
veterinarian.
Histopathologic Description:
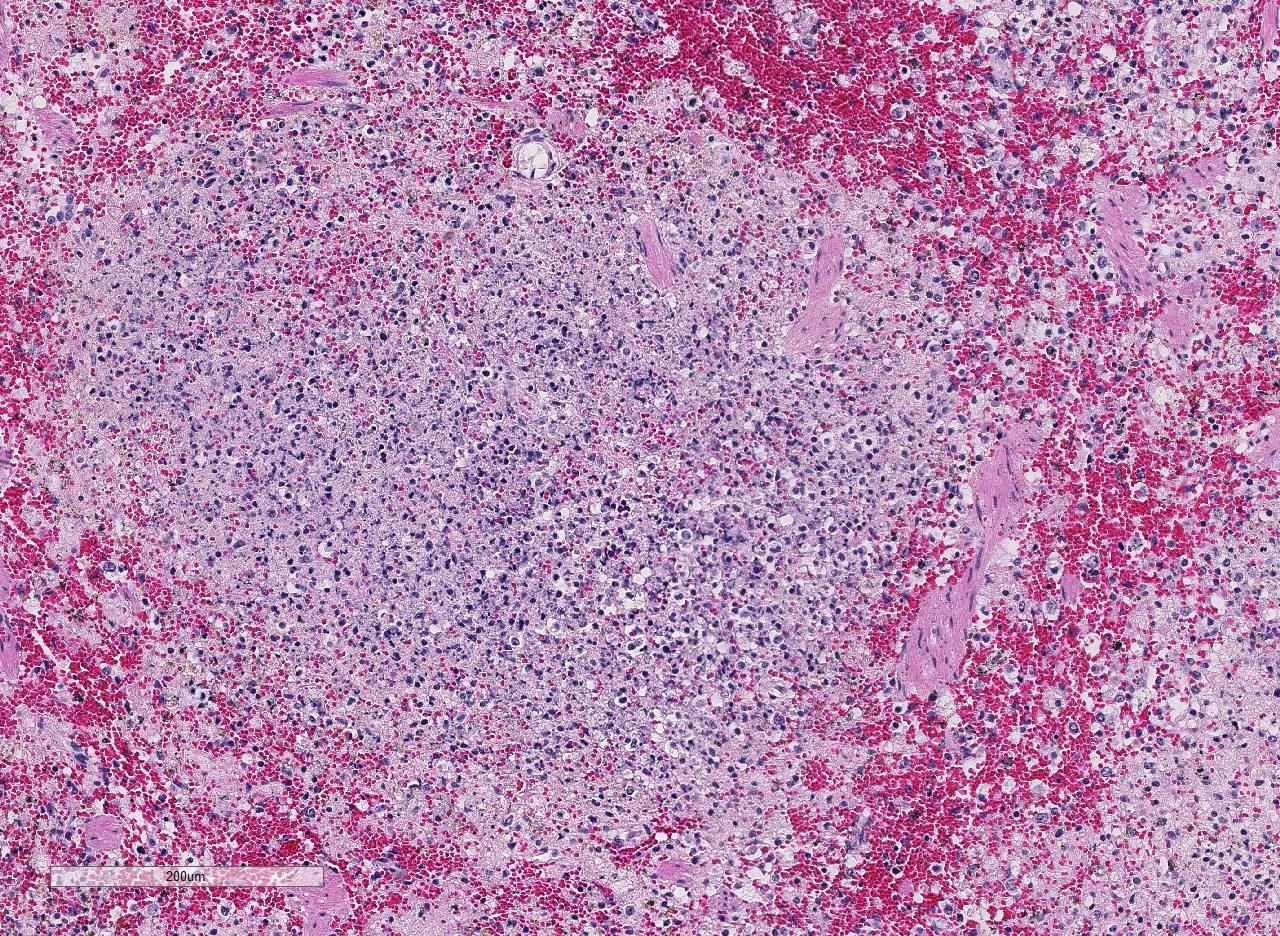
In sections of
spleen, there is disseminated and coalescing
necrosis of germinal centers which extends
into adjacent red pulp. Foci of necrosis are
accompanied by an inflammatory response
comprised of neutrophils and macrophages. By use of special technique, numerous small
gram-negative coccobacilli are seen within
necrotic foci and the cytoplasm of individual
inflammatory cells.
Morphologic Diagnosis:
Severe, subacute, multifocal, coalescing,
necrotic, and pyogranulomatous splenitis
Lab Results:
Francisella tularensis
was isolated from the spleen and from a
lymph node sample.
Condition:
Pyogranulomatous splenitis/Francisella tularensis
Contributor Comment:
Francisella
tularensis is a gram-negative, facultative
intracellular pathogen.
5 F. tularensis is
subdivided into two subtypes. Type A is F.
tularensis subsp. tularensis and has and
infectious dose in humans of <10 CFUs,
whereas type B is F.
tularensis subsp.
holarctica which as an infectious dose of
<103 CFU and a milder form of tularemia in
humans.
5 The organism is abundant in nature
and infects many mammalian and arthropod
species.
7 F.
tularensis type A has been
isolated from cats on numerous occasions
and can be transmitted from cats and other
animals (deer, personal experience) to
humans.
1,3,7
Diagnosis, in some cases, may be difficult,
but culture appears to be more sensitive than
immunohistochemistry.
7 Gross lesions
consist of multiple pinpoint white foci on
the spleen, liver, and lymph nodes. As a
facultative intracellular parasite, it may
persist for years as a latent infection.
7
The
genes for several virulence factors have been
identified and shown to share some features
with the intracellular parasite,
Listeria
monocytogenes.
4
Tularemia in other
mammalian species such as horses and
sheep are often associated with heavy
infestation by ticks such as
Dermacentor
andersoni and
Amblyomma americanum.
7
One serologic survey indicated 12 24
percent of cats had antibodies to F.
tularensis due to natural exposure.
6
Those
serologically positive animals were negative
for F.
tularensis DNA, indicating infection
may be may have been cleared naturally.
Tularemia should be considered in a
differential diagnosis of unexplained febrile
illness in cats.
JPC Diagnosis:
Spleen: Splenitis,
necrotizing, multifocal to coalescing, severe,
with mild lymphoid depletion and fibrin
deposition, domestic shorthair cat, Felis
catus.
Conference Comment:
The contributor
provides a great example of typical lesions
of
Francisella tularensis. In cats, there often
is severe systemic disease and pathological
manifestations are dependent on the
dissemination of the pathogen.
1,8,9 As
mentioned by the contributor, classic gross
lesions for tularemia are miliary white foci
2mm or more in diameter in the liver,
spleen, and lymph nodes. Histologically, the
lesions are characterized by focal areas of
severe necrosis, as seen in this case.
8 This
gram-negative, intracellular bacillus can
infect humans, wild rabbits, rodents, and
over 100 species of wild and domestic
mammals, birds, fish, and reptiles.
3,9 In the
North America, the wild rabbit is the
reservoir for the biovar tularensis (type A).
Biovar holarctica (type B) is more common
in aquatic species such as beavers and
muskrats.
F. tularensis biovar mediasiatica
and F. novicida are restricted to central
Asia.
7,8 Sporadic outbreaks of tularemia are
known to occur in sheep and foals in
association with heavy infestation with
Dermacentor andersoni and Amblyomma
americanum ticks. Typically, enlargement of
the liver, spleen, and kidneys with miliary
foci of necrosis are seen on post-mortem
examination.
8 Dogs are generally highly
resistant to natural infection, but there have
been rare reports of mild disease in canines.8
The most common route of infection for
humans originates from cleaning and
skinning infected rabbits as well as
arthropod bites. Humans can also be
infected via contaminated water supplies
and consumption of undercooked meat.
6 In
addition to natural infection,
F. tularensis is
considered to be a serious potential
bioterrorism agent, because it is one of the
most infectious pathogenic bacteria known.
As mentioned by the contributor, inhalation
of as few as 10 organisms can cause severe
pneumonic tularemia disease leading to
serious illness and death.
1,6
Experimentally-induced lesions from
inhalation in African green monkeys
included necrotizing pyogranulomatous
lesions which targeted the lung and
lymphoid tissue in addition to disseminated
miliary necrotic foci on multiple organs and
moderate to marked lymphoid depletion of
the splenic white pulp and mediastinal
lymph nodes.
6 Conference participants
agreed that in this case, both red and white
pulp of the spleen are affected by necrosis;
however, lesions generally centered on the
white pulp and extended into the red pulp in
conjunction with severe lymphoid depletion
and lymphocytolysis. There are currently no
vaccines available to prevent disease.
6 As a
result, conference participants discussed that
extreme care needs to be taken when dealing
with and shipping suspect tularemia cases.
These facultative intracellular organisms are
most commonly located within macrophages,
but may also be present
extracellularly in exudates and necrotic
debris. The organisms can also infect and
survive in dendritic cells, neutrophils,
hepatocytes, and lung epithelial cells. The
ability of
F. tularensis to infect
macrophages, evade the immune system by
preventing phagolysosome fusion, rapidly
replicate within macrophages, and
disseminate widely throughout the body is
the key to its pathogenesis.
1,7,8
References:
1. Brotcke A, Weiss DS, Kim CC, et al.
Identification of Mg1A-regulated
genes reveals novel virulence factors
in
Francisella tularensis.
Infect
immune. 2006; 74:6642-6655.
2. Gyuranecz M, Szeredi L, Makrai L
et al. Tularemia of European Brown
Hare (
Lepus europaeus): A
pathological, histopathological, and
immunohistochemical study. Vet
Pathol. 2010; 47(5):958-63.
3. Inzana TJ, Glindemann GE, Snider
G, et al. Characterization of a wildtype
strain of Francisella tularensis
isolated from a cat.
J Vet Diagn
Invest. 2004; 16:374-381.
4. Magnarelli L, Levy S, Koski R.
Detection of antibodies to
Francisella tularensis in cats.
Res
Vet Sci. 2007; 82:22-26.
5. Pechous RD, MCCarthy TR, Zahrt
TC. Working toward the future:
Insights into
Francisella tularensis
pathogenesis and vaccine
16
development. Microbiol
Mol Biol
Rev. 2009; 73:684-711.
6. Twenhafel NA, Alves DA, Purcell
BK. Pathology of inhalational
Francisella tularensis spp. tularensis
SCHU S4 infection in African green
monkeys (Chlorocebus
aethiops).
Vet Pathol. 2009; 46:698-
706.
7. Valentine BA, DeBey BM, Sonn RJ,
Stauffer LR, Pielstick LG. Localized
cutaneous infection with
Francisella
tularensis resembling ulceroglandular
tularemia in a cat.
J Vet
Diagn Invest. 2004; 16:83-85.
8. Valli VEO. Hematopoietic system.
In: Maxie MG, ed. Jubb, Kennedy,
and Palmer's Pathology of Domestic
Animals. Vol 3. 6th ed. Philadelphia,
PA:Elsevier; 2016:184-186.
9. Weinberg AN, Branda JA. Case 31-
2010: A 29-year-old woman with
fever after a cat bite.
N Engl J Med.
2010; 363:1560-1568.