WSC 22-23
Conference 11
Case III:
Signalment:
5 year old, female, African spurred tortoise, Geochelone (Centrochelys) sulcata, C. sulcata
History:
Animal kept as pet by a private. No information regarding general state of health, past medical history and gross post mortem findings were given by the referring private veterinary practitioner. Only a formalin fixed liver was submitted for histology.
Gross Pathology:
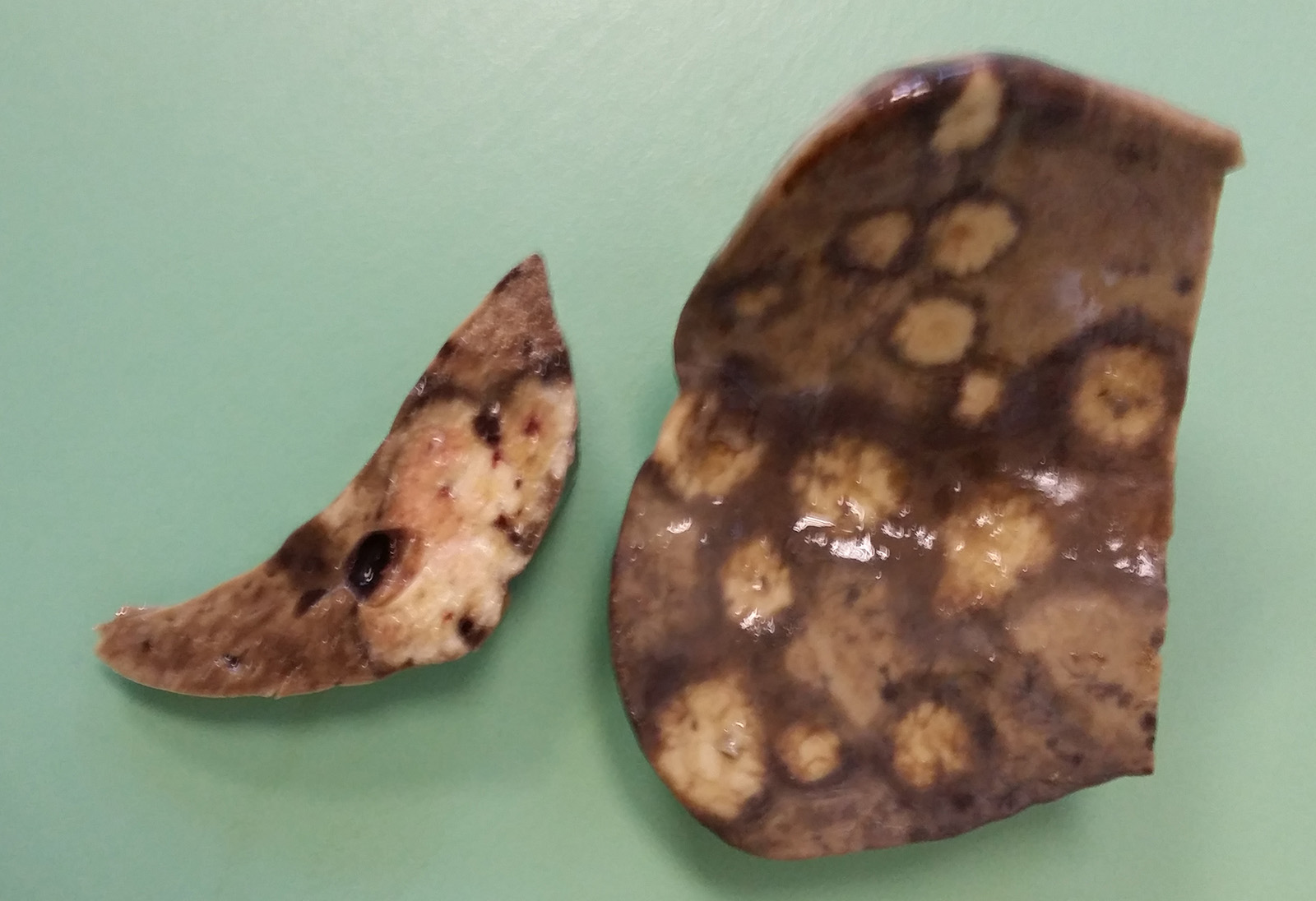
The liver was moderately increased in volume, with rounded margins and diffusely brown to yellow in color. On the surface and in cross-section, multiple to confluent, round and white to yellow lesions was present (multifocal necrotizing hepatic foci). The lesions are smooth with moderately irregular margins and are surrounded by a hyperemic halo. In cross section, hemorrhages are present in the center of the necrosis. The consistency of the liver is markedly reduced, and the organ is friable to the manipulation.
Laboratory Results:
No findings reported.
Microscopic Description:
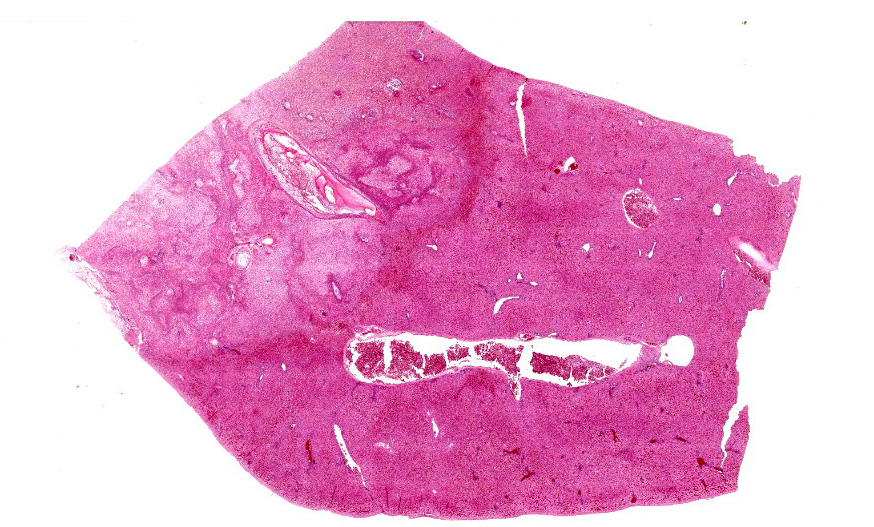
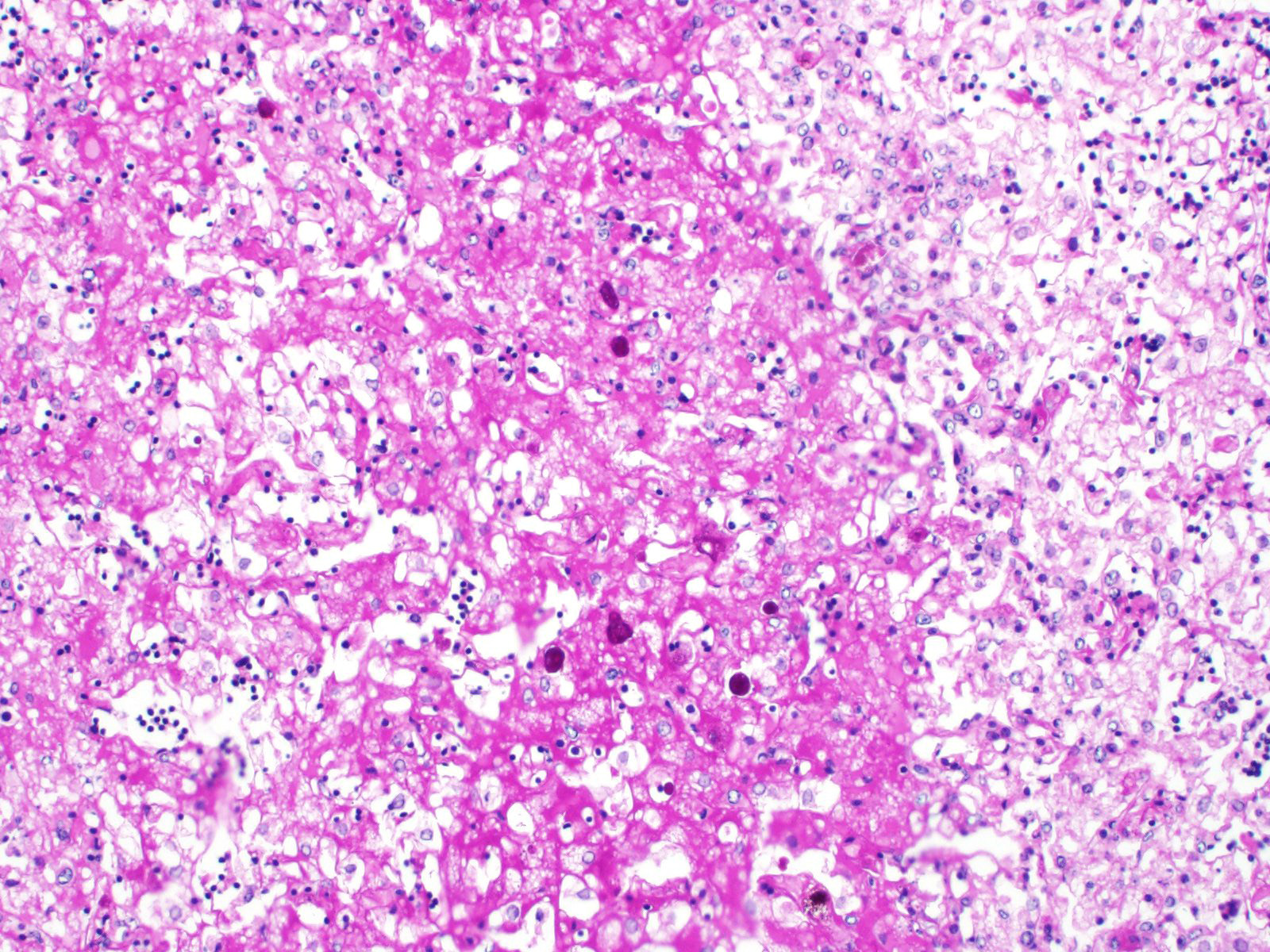
Liver: Approximately 30% of the hepatic parenchyma is characterized by multifocal, random, 1-3 mm in diameter, irregularly nodular, areas of necrosis and heterophilic-histiocytic inflammation.
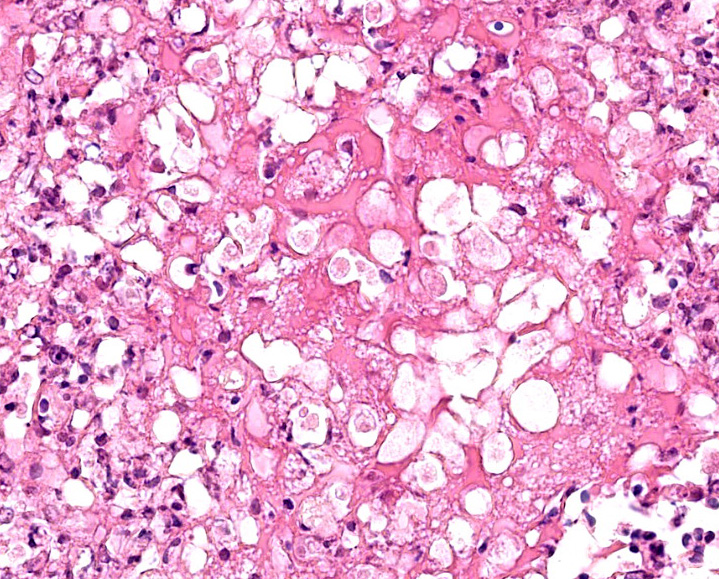
The centre of the lesions is composed of abundant basophilic granular karyorrhectic/necrotic debris with complete loss of cellular details (lytic necrosis) admixed to intensely eosinophilic, shrunken hepatocytes with lysed nuclei (coagulative necrosis). Elevated numbers of both viable and degenerated heterophils with karyolitic nuclei can be seen admixed with the necrosis and at the periphery of the necrotic foci that are multifocally also associated at the periphery with a lightly eosinophilic fibrillary, finely beaded, meshwork (fibrin).
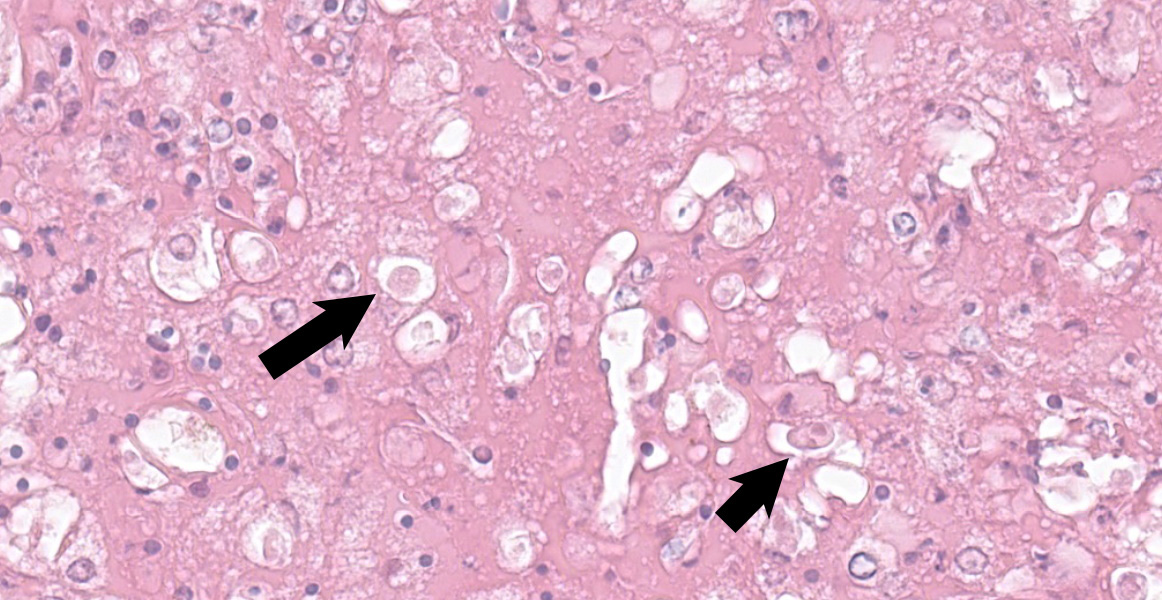
At the periphery of the necrotic foci, surrounding and invading vessel walls and occasionally in the cytoplasm of macrophages variable numbers of oval (15-30 µm in diameter) protozoal structures can be seen. Parasites have a distinct cell membrane, a finely granular to vacuolated cytoplasm, and a single nucleus with marginated chromatin and a lightly basophilic central karyosome (amoebic trophozoites).
The hepatic parenchyma is multifocally characterized by swollen hepatocytes, with granular to clear cytoplasm (vacuolar/hydropic degeneration). Multifocally in the cytoplasm of hepatocytes, yellow-greenish granular pigment (bile stasis) is evident.
Fibrin thrombi occasionally entrapping trophozoites are visible in vascular lumens with endothelial cell necrosis. Diffusely sinusoidal hyperemia is present.
Contributor’s Morphologic Diagnoses:
Liver: severe, multifocal to coalescing, random, acute to subacute necrotizing hepatitis and vasculitis with perivascular and intralesional trophozoites consistent with Entoamoeba spp.
Contributor’s Comment:
Entamoeba invadens is the most important amoeba species infecting reptiles.6,14,18 This protozoan parasite belongs to the phylum Sarcomastigophora, subphylum Sarcodina (Rhizopoda), order Amoebida, family Entamoebidae, genus Entamoeba and is similar morphologically to Entamoeba histolytica. Although, E. invadens may be the main parasite involved in reptile pathology, other Entamoeba species, such as E. terrapinae, E. insolita, E. barreti, E. testudinis and E. ranarum, can also infect reptiles8.
E. invadens is a common commensal parasite of different free-ranging reptiles including snakes, crocodilians, turtles, tortoises, and lizards.18 Turtles, tortoises, and crocodiles are considered reservoir species for E. invadens. Herbivorous tortoises harbor the parasite and only occasionally demonstrate clinical signs.6,11 The mechanism of this resistance is unknown. Based on data available for Acanthamoeba, it is possible that some types of sugars (mannitol and glucose) can prevent the adhesion of the trophozoite to the mucosal surface. Among tortoises, giant tortoises (Geochelone spp.)11 and the northern map turtle (Graptemys geographica)17 seem to be more susceptible. Snakes which develop amebiasis are Boidae (boas), Pythonidae (pythons), Crotalidae (crotalids), Elapidae (colubrids), and Viperidae (vipers). Snakes resistant are greater snake and northern black racers. All the crocodile seems to be immune to E. invadens.4 The presence of carnivorous reptiles resistant to the infection has been associated with the evolutionary strategy to feed with snakes.4 Among lizards, Varanidae (monitor lizard) seems to be the most susceptible species. The onset of clinical signs in resistant animals has been correlated with stress, age and immunosuppression.8
Hepatic amoebiasis in tortoises is rare and an outbreak of severe entero-hepatic disease with duodenal involvement seems to have been reported once.9 In snakes and lizards, E. invadens is responsible for a severe epizootic disease characterized by necro-hemorrhagic gastroenteritis, colitis, or entero-hepatitis. Occasionally, the parasite can invade the bloodstream and reach other organs causing extraintestinal complications. Atypical amebiasis manifesting as myositis and ulcerative dermatitis has been reported in a common water monitor lizard (Varanus salavtor).2
E. invadens has a worldwide distribution2, with a life cycle characterized by two morphologically different stages, a motile trophozoite stage (vegetative form) and a cystic stage (resistant form). Because of the protection conferred by their walls, amebic cystic forms can survive days to weeks in the environment. In asymptomatic carriers, trophozoites are confined to the intestinal lumen (non-invasive infection). In some animals, trophozoites acquire invasiveness, infiltrating the intestinal wall and the biliary ducts, inducing the enteric form (intestinal disease) or the systemic form (extraintestinal disease). In snakes and lizards, the liver is considered the major extraintestinal target organ.7 In snakes, invasion of lungs, spleen, pancreas, kidneys, and subcutaneous tissues occurs via hematogenous route.9
The pathogenic transformation of the trophozoite into an invasive parasitic form has not been elucidated for E. invadens. Although a parallel with the pathogenesis of E. histolytica in human beings has been hypothesized explaining the different forms via the recognition of different parasite strains.19
The parasite has an oro-fecal cycle, mature amoebal cyst are ingested with contaminated water, food or arthropod vectors (flies, roaches). Excystation occurs in the small intestine with the release of a trophozoite characterized by four nuclei that after division produce four trophozoites with a single nucleus. Each of these 4 zoites divides in 2, producing a total of 8 trophozoites (amebulae). Trophozoites are the mobile and labile form of the parasite and are capable of migrating in the large intestine. Proteolytic enzymes (glycosidases such as galactosidase, mannosidase, fucosidase and others) produced by trophozoites disrupt the mucous barrier and parasites are able to attach to the gastrointestinal epithelium. The trophozoite first adheres to the intestinal mucin layer and to epithelial cells by a surface Gal/GalNAc-specific lectin (adhesin), and releases pore-forming polypeptides called amoebapores that are small potent peptides able to induce lysis of host cell membrane resulting in intracellular calcium elevation and eventual cell death. After the epithelial cells are lysed, there is colonic gland invasion and cysteine proteases degrade the extracellular matrix so that trophozoites burrow into lamina propria invading the submucosa5. In the large intestine trophozoites start dividing by binary fission producing cysts released in the environment. In humans, amoebic liver abscess (amoeboma, ALA=Amebic liver abscess) is the most frequent extraintestinal manifestation of E. histolytica infection. The liver can be infected by two ways, by common biliary duct invasion (continuity ascending route), or by portal vein invasion (thromboembolic route).
Virulence factors involved in ALA development include those necessary for complement resistance (PPGs), ROS resistance (peroxiredoxin), lysis (CPs and amoebapores), and cell adherence (notably, KERP1 and the Gal/GalNAc lectin).17
In a recent study on E. histolytica pathogenesis, amoebae were demonstrated to kill by biting off and ingesting distinct fragments of viable cells. The internalization of bites of living human cells is reminiscent of trogocytosis (Greek trogo-, nibble) observed between immune cells, but amoebic trogocytosis differs since it results in death.13
Clinical signs associated with amoebiasis in reptiles include anorexia, weight loss, mucoidal or hemorrhagic diarrhea, dehydration, and death after weeks or month of illness. Sudden death is also commonly reported.4,16,11
Typical gross findings are thickening of the gastrointestinal wall, with ulcerative and necrotizing colitis in snakes and duodenitis in turtles. Lesions can extend to other parts of the gastrointestinal tract.2,9 Tubular organs can have transmural ulceration. Ulcers may be flask shaped, with a narrow neck and broad base. The lumen of the intestine is filled with blood, necrotic debris, and mucus. Extraintestinal lesions are secondary to the intestinal infection, and all organ systems can potentially be affected. The lesions start as abscesses evolving in necrotizing lesions centered on blood vessels.2 Secondary bacterial infections are commonly found in liver and gastrointestinal tract.
Typical histological findings are necro-hemorrhagic lesions in all organs affected. Usually, amoebae can be found admixed to the necrotic debris. E. invadens trophozoites range from 10 to 21 µm (intestine: 12-19 x 10-13 µm, liver: 13-21 x 11-18 µm).9 Nucleus is 3-5 µm in diameter, round and periphery located. A single central endosome is present, and it is 0.6-1 µm. E. invadens cysts range from 11 to 20 µm and have four nuclei.
Ultrastructurally,3 trophozoite cytoplasm is filled by numerous, variably sized vacuoles. Vacuoles are surrounded by an electron dense membrane and contain starch granules and bacteria (food vacuoles). Cytoplasmic, irregularly shaped, electron dense aggregates of 200 nm are associated with the food vacuoles and the nucleus (chromatoid particles). Inside the nucleus, a single electron dense nucleolus is usually present. Cyst cytoplasm can be divided into two areas, one electrondense adjoining the nuclei and around the cell wall and containing vacuoles, and one electrolucent, in between, containing glycogen. The chromatoid particles present in the cyst, are usually aggregated in large crystalline structures (chromatoid bodies).3
Gross and histopathological finding are distinctive and mostly diagnostic, additional diagnostic tools are: fecal flotation, histochemical stains (PAS staining-purple, GMS staining-black, and Heidenhain’s iron strain for chromatin), immunohistochemistry, indirect immunofluorescence and PCR.1,18
Other infectious agents causing hepatic necrosis and hepatitis in tortoises include: Herpes viruses10 (i.e. tortoise herpesvirus type 1 and 2- responsible for sudden death, upper respiratory disease, hepatic disseminated necrosis and intranuclear eosinophilic inclusion bodies; siadenovirus16 (systemic infection, intranuclear basophilic to amphophilic inclusion bodies in hepatocytes and non suppurative hepatitis), Iridoviruses (i.e. gen. Ranavirus: disseminated hepatic necrosis, basophilic inclusion bodies, vasculitis)10; Bacteria, mostly in septicemic infections or following a primary viral disease (i.e. Aeromonas spp. Escherichia coli, Pasteurella testudinis, Morganella morganii, Serratia marcescens Chlamydophila spp.10 and Fungi causing sistemic mycoses such as Aspergillus, Paecilomyces, and Penicillium spp. can sporadically induce hepatic lesions.10
Contributing Institution:
DIMEVET-Anatomical Pathology Section
Via Celoria 10
20133 Milano, Italy
JPC Diagnosis:
Liver: Hepatitis, necrotizing and embolic, focally extensive, marked, with vasculitis and numerous amoebic trophozoites.
JPC Comment:
A variety of pathogenic and commensal Entamoeba spp. infect the gastrointestinal tract of vertebrates and invertebrates, and the contributor provides a thorough summary of Entamoeba infection in reptiles. In addition to E. invadens, snakes may rarely be susceptible to another Entamoeba species - Entamoeba ranarum, which typically infects but rarely causes disease in amphibians.12,14 There have been two published reports of E. ranarum infection causing necrotizing colitis in snakes; one in a ball python, and one in a boa. While both cases had colitis similar to that caused by E. invadens, neither featured spread to other organs, such as the liver.12,14 In one case, the snake had previously been housed with frogs, so fecal-oral transmission is hypothesized. 12
Another Entamoeba afflicting amphibians has recently been identified in invasive cane toads (Rhinella marina) in Australia. These amphibians were introduced in 1935 as an unsuccessful means to control beetles consuming sugar cane crops and now inhabit a range of over 1 million square kilometers.16 In 2014, researchers discovered the novel Entamoeba during an outbreak of severe colitis and high mortality in cane toads in one region of northern Australia. PCR and subsequent sequencing illustrated its close relation to both E. ranarum and E. invadens and lead to its classification is Entamoeba sp. CT1.20 Affected toads had either no gross lesions or mild prominence of vasculature and thickenning of intestinal walls. Histologically, all affected toads had some degree of colitis, with the most severe cases featuring deep ulcers with edema and fibrosis. Entamoeba trophozoites were associated with the affected mucosal epithelium and ulcers, but there was no evidence of spread outside the gastrointestinal tract.20 Subsequent research to evaluate the impact on native frog species has demonstrated a prevalence of approximately 24% in cane toads in the same region and no identification of the parasite in any of the 11 tested native frog species.16 While the authors acknowledge that intermittent shedding, sample degradation, and testing limiations may have affected the abiilty to isolate the parasite, the results are encouraging for native species and anuran conservationists.16
References:
- Bradford CM. Denver M and Cranfield MR. Development Of A Polymerase Chain Reaction Test For Entamoeba invadens. J Zoo Wild Med. 2008; 39(2): 201-207.
- Chia M.Y., Jeng C.R., Hsiao S.H., Lee A.H., Chien C.Y., and Pang V.F. Entamoeba invadens Myositis in Common Water Monitoring Lizard (Varanus salvator). Vet Path. 2009; 46:673-676.
- Deutsch K, Zaman V. An Electron Microscopic Study of Entamoeba Invadens Rodhain 1934. Exp Cell Res. 1959;17(2): 310-319.
- Divers SJ. Parasitic diseases of the Reptiles. The Merck Veterinary Manual http://www.merckvetmanual.com/mvm/exotic_and_laboratory_animals/reptiles/parasitic_diseases_of_reptiles.html
- Espinosa-Cantellano M, Martínez-Palomo A. Pathogenesis of intestinal amebiasis: from molecules to disease. Clin Microbiol Rev. 2000;13(2):318-31.
- Flannagan J. P. Chapter 4. Fowler's Zoo and Wild Animals Medicine. Vol 8. 70.
- Garate M, Cubillos I, Marchant J., and Panjwani N. Biochemical Characterization and Functional Studies of Acanthamoeba Mannose-Binding Protein. Infect Immun. 2005; 73(9):5775-81.
- García G, Ramos F, Pérez RG, Yañez J, Estrada MS, Mendoza LH, Martinez-Hernandez F, Gaytán P. Molecular epidemiology and genetic diversity of Entamoeba species in a chelonian collection. J Med Microbiol. 2014;63(Pt 2):271-83.
- Jacobson E, Clubb S, Greiner E. Amebiasis in red-footed tortoise. JAVMA. 1983; 183(11): 1192-1194
- Jacobson ER. Infectious Diseases and Pathology of Reptiles: Color Atlas and Text. Boca Raton, FL: CRC/Taylor & Francis; 2007: pp. 398, 404, 531.
- Klingenberg RJ. Understanding Reptile Parasites, 2nd edition. Chapter 11, Amoebiasis.
- Michaely LM, von Dornberg K, Molnar V. Entamoeba ranarum Infection in a Ball Python (Python regius). J Comp Path. 2020; 179: 74-78.
- Ralston KS, Solga MD, Mackey-Lawrence NM, Somlata, Bhattacharya A, Petri WA Jr. Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. 2014; 24;508(7497):526-30.
- Richter B., Kübber-Heiss A., Weissenböck H. Diphtheroid colitis in a Boa constrictor infected with amphibian Entamoeba Vet Parasitol. 2008;153(1-2):164-7.
- Rivera S, Wellehan JF Jr, McManamon R, Innis CJ, Garner MM, et al. Systemic adenovirus infection in Sulawesi tortoises (Indotestudo forsteni) caused by a novel siadenovirus. J Vet Diagn Invest. 2009;21(4):415-26.
- Rivory P, Brown G, Shilton C, Shine R, Slapeta J. Apparent lack of spill-over of parasites from an invasive anuran: PCR detects Entamoebain cane toads (Rhinella marina) but not in sympatric Australian native frogs. Int J Parasitol Parasites Wildl. 2020(12):207-213.
- Santi-Rocca J, Rigothier MC, Guillén N. Host-microbe interactions and defense mechanisms in the development of amoebic liver abscesses. Clin Microbiol Rev. 2009 ;22(1):65-75.
- Scullion F.T., M. Scullion G. Gastrointestinal Protozoal Diseases in Reptiles. Exot. Pet Med. 2009; 18(4), 266-278.
- Sehgal D., Bhattacharya A., Bhattacharya S.. Pathogenesis of infection by Entamoeba histolytica. J Biosci. 1996; 21(3): 423-432.
- Shilton CM, Slapeta J, Shine R, Brown GP. Pathology Associated with an Outbreak of Entamoebiasis in Wild Cane Toads (Rhinella marina) in Tropical Australia. Vet Pathol. 2019; 56(6):921-931.