Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 8
23 October 2019
Dr. Dalen Agnew, DVM,
PhD, DACVP
Associate Professor
Pathobiology and Diagnostic Investigation
Michigan State University
College of Veterinary Medicine
East Lansing, Michigan
CASE III: VS17005 3 (JPC 4102155).
Signalment: 9-year-old, female rhesus macaque (Macaca mulatta)
History: Multiple late term abortions occurred in a large dairy goat herd. Submitted samples included fixed tissues, tissue pools, abomasal fluid and serum from one of the aborting dams.
Gross Pathology: This animal received 4Gy whole body irradiation three years prior to presentation. It had a history of menorrhagia and anemia (Hct 14.8%). A hysterectomy was performed to prevent further blood loss. During the procedure the uterus was found to be adhered to the right side of the abdominal wall.
Laboratory results: Placentas were positive for Chlamydophila by antigen ELISA. PCR for Toxoplasma gondii was negative on placenta and brain. No Campylobacter was isolated and PCR for BVD was negative. FA for Leptospira was negative. A single dam serum was positive for antibodies to Coxiella but negative for Brucella abortus, BVDV and Toxoplasma. Immunohistochemistry for Coxiella burnetii on the placenta was positive.
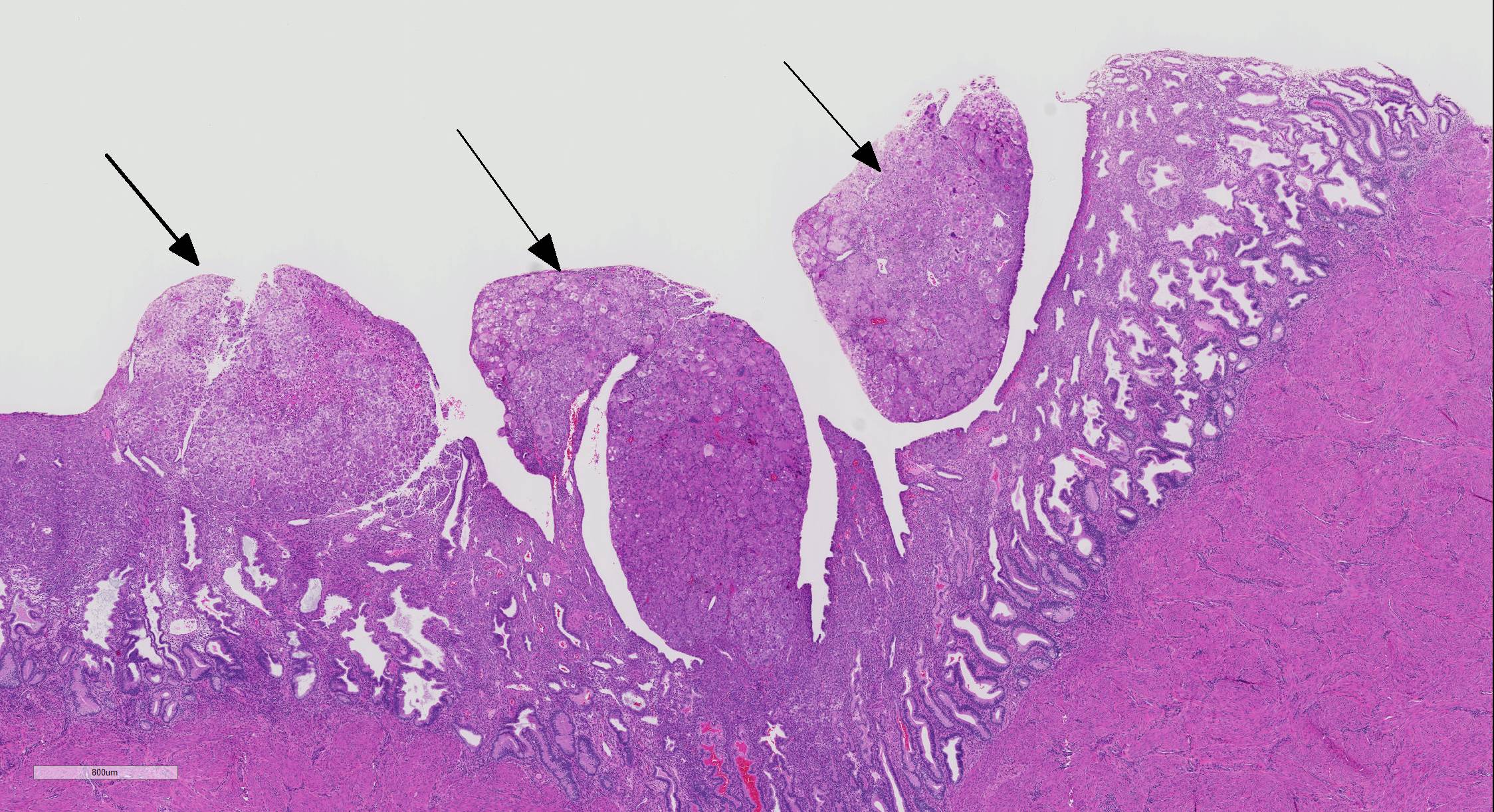
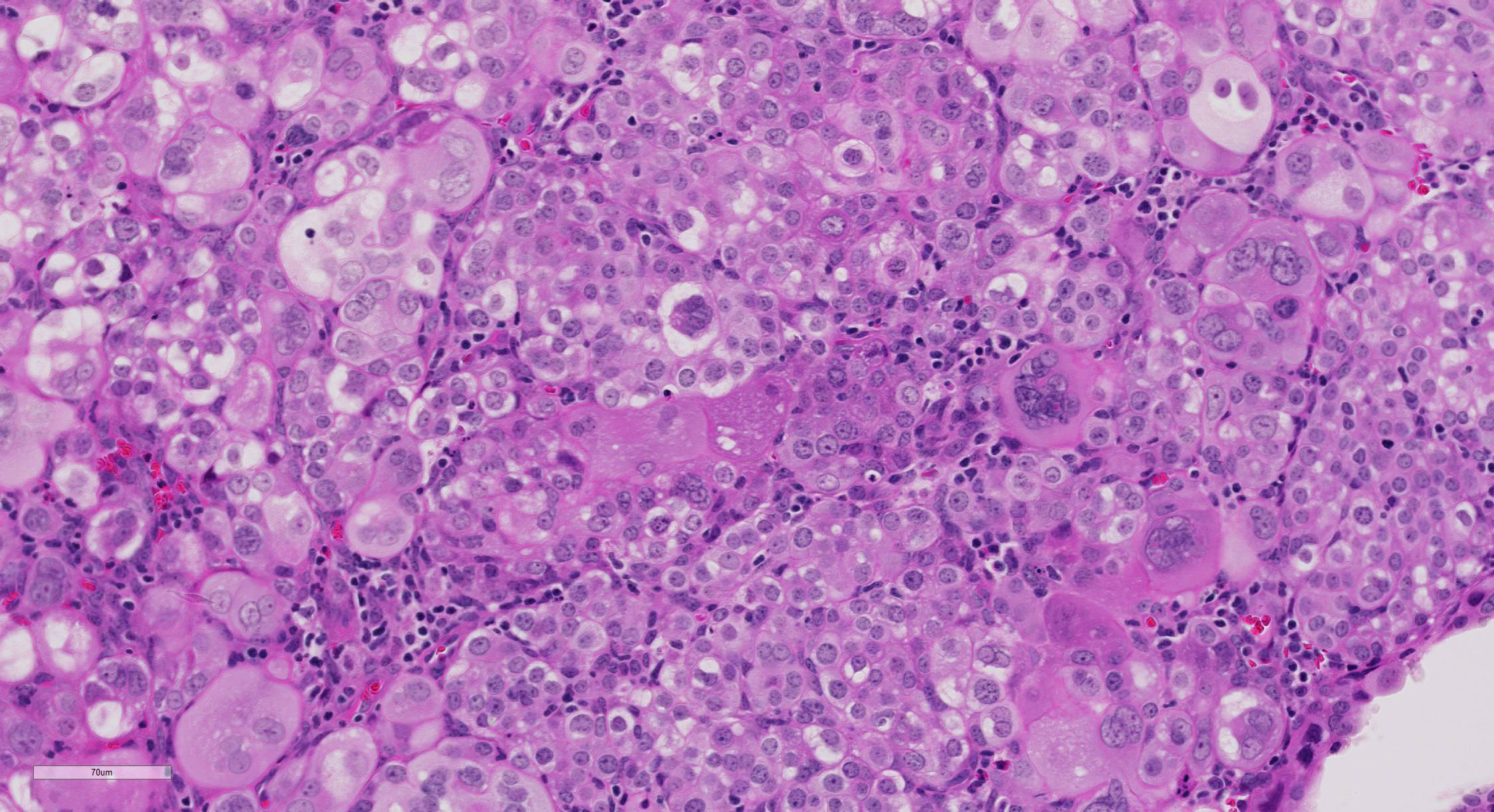
Microscopic Description: The superficial endometrium is expanded and effaced in areas by an unencapsulated, poorly demarcated neoplasm composed of sheets of pleomorphic neoplastic cells supported by fine stroma, which projects into the lumen in some areas. Aggregates of macrophages, lymphocytes and few eosinophils and granular leukocytes are scattered throughout. The neoplastic cells have variably distinct cell borders, are round to polygonal, have abundant eosinophilic vesicular cytoplasm, 1-12 oval nuclei with stippled chromatin, and 1-2 nucleoli. Mitoses are rare. A few cells, especially towards the lumen, have undergone degeneration and necrosis. The remaining endometrium has few scattered dilated glands lined by epithelial cells with basally arranged nuclei, without mitoses.
Contributor Morphologic Diagnosis: Uterine trophoblastic tumor
Contributor Comment: Gestational trophoblast disease (GTD) is a rare disease of pregnancy, a result of abnormal differentiation of trophoblasts, but may also rarely develop from germ cells in the absence of pregnancy.13 Since trophoblasts lack the common regulatory pathways that prevent the development of neoplasms, either molar gestations/hydatiform moles or trophoblastic tumors can develop.16 Hydatiform moles are abnormal pregnancies characterized by aberrant chromosomal changes. Women greater than forty years of age and younger than 20 are more likely to develop molar gestations/hydatiform moles. Uterine epithelioid trophoblastic tumors have been reported in a red-tailed guenon (Cercopithecus ascanius)4 and an African green monkey (Chlorocebus aethiops sabaeus).2 Ovarian nongestational trophoblastic tumors (2 choriocarcinomas and 1 epithelioid trophoblastic tumor) have been reported in captive macaques.6,7,10,15
Trophoblast tumors can be classified into one of three subcategories: choriocarcinomas, placental site trophoblastic tumors, and epithelioid trophoblastic tumors. Choriocarcinomas are malignant tumors composed of bilaminar cytotrophoblasts and syncytiotrophoblasts without chorionic villi. In some cases, these represent a component of mixed germ cell tumors of the ovary. Pure nongestational choriocarcinomas are extremely rare in humans. Typically the neoplastic cells are strongly positive for human chorionic gonadotrophin (β-hCG) and weakly positive for human placental lactogen (hPL).14 Placental site trophoblastic tumors are composed of sheets of variably sized trophoblasts with single to multiple nuclei with atypia. These cells may permeate the myometrium and blood vessels.3 The cells are usually positive for hPL and variably positive for β-hCG and placental-like alkaline phosphatase (PLAP).9,14 Epithelioid trophoblastic tumors are composed of sheets of monomorphic intermediate trophoblasts which resemble the chorionic membrane.3 The cells are variably immunoreactive for placental-like alkaline phosphatase (PLAP) and hPL but strongly immunoreactive for E-cadherin and epidermal growth factor receptor.9,14
The clinical signs associated with GTD vary widely. Symptoms usually present within the first trimester and include bleeding and anemia, a larger than normal uterus for the gestational age, hyperemesis, pre-eclampsia, hyperthyroidism and respiratory distress. Abnormal fetal organs observed by ultrasonography and markedly elevated serum hCG can aid in the clinical diagnosis. Histopathology and immunohistochemistry provides a confirmatory diagnosis. The treatment options of trophoblast tumors depend on the stage of the tumor. 13,16
Contributing Institution:
Wake Forest School of Medicine
Department of Pathology, Section on Comparative Medicine
Medical Center Boulevard \ Winston-Salem, NC 27157
JPC Diagnosis: Uterus: Choriocarcinoma
JPC Comment: The contributor has presented a concise yet informational description of a confusing set of neoplasms that are extremely rare in all species. These tumors are a subset of gestational trophoblastic disease, which as stated above, includes hydatiform moles.1
Hydatiform moles (hydatiform meaning cystic, mole simply
referring to a clump of growing tissue) arise during pregnancy from non-viable eggs
that implant in the uterus, and are the most common of the gestational
trophoblastic diseases. These eggs have lost the nucleus, and hence the
maternal DNA. The mole, a parthenogenetic zygote, results from fertilization
by a spermatozoa undergoing mitosis following combining with the egg (resulting
in a 46 XX phenotype) or alternatively two haploid spermatozoa (resulting in a
46 X,Y karyotype.)1 Even more rarely a normal egg may be fertilized
by two sperm which reduplicate themselves (resulting in a 69, XXY karyotype) â
known as a partial mole. Partial moles, due to the presence of maternal DNA
may have discernable, but disorganized fetal elements within their structure.1
Moles may often be grossly differentiated from true trophoblastic tumors grossly
and histologically by their grapelike appearance (owing to their development of
chorionic villi, not seen in the true trophoblastic tumors. and histologically,
but the presence of chorionic villi, which are not present in the true
trophoblastic tumors. They may be invasive, growing into the wall of the
uterus, or actually turn into choriocarcinomas (this happens into 4% of
tumors.)1
The true trophoblastic tumors, as mentioned by the contributors, include
choriocarcinoma, the placental site trophoblastic tumor (PSTT), and epithelioid
trophoblastic tumor (ETT), and their differentiation histologically is based on
morphology and immunohistochemistry (reviewed above in the contributorâs
comments.)12 Choriocarcinomas are by far the most malignant and
common of the three, often demonstrating significant invasion of the uterus and
distant metastasis in approximately 40% of human cases. The other two tumors
are far less common and both are derived from the intermediate trophoblast.
PSTT is the rarest subtype with 1 per 100.000 pregnancies.9 These
are slow growing malignancies that most affect women of reproductive age and
are seen following a normal or ectopic pregnancy or miscarriage, with rare
cases affecting post-menopausal women. Because of its similarities to a normal
implantation site, it was initially called a "placental pseudotumor".9
The cellular population of the placental site trophoblastic tumor is
monomorphic as composed to the dimorphic population of choriocarcinomas, and
its immunohistochemical profile is the reverse, with strong staining for hPL
and weak staining for hCG (as the choriocarcinoma stains strongly for hCG and
weakly for hPL.11 Interestingly, the differential levels of these
hormones are often used as a clinical test for these particular neoplasms as
well. The third neoplasm, epithelioid trophoblastic tumor (ETT) was first
diagnosed in humans by Shih and Silverberg in 19982, and is also a
neoplasm of intermediate trophoblasts (as is the PSTT) but resemble those of
the chorionic villi, rather than the placental site (as seen with the PSTT). The
neoplastic cells stain weakly for hCG and HPL, but strongly for cytokeratin and
inhibinâA, epithelial growth factor, and e-cadherin.2 A review of
the immunohistochemical staining patterns for the various types of gestational
trophoblastic diseases is presented in Table 1.
Since the submission of this case, this case has also been presented at the
2018 Satellite Sympoisum of the National Toxicology Program at the annual
meeting of the Society of Toxicologic Pathologists, the minutes of which were
subsequently published in Toxicologic Pathology. The presenter
described the immunohistochemical findings for this neoplasm including a
general immunpositivity for hCG and strong positivity in the multinucleated
cells. The cells stained diffusely positive for pancytokeratin, but failed to
stain for human placental lactogen, placental alkaline phosphatase, and p63.
There were rare foci of CD10 immunopositivity, and these cells failed to stain
with hCG and pancytokeratin. Based on these findings, the contributor and the
participants at the conference prefer a diagnosis of choriocarcinoma. This is
in agreement with the immunohistochemical stains run at the Joint Pathology
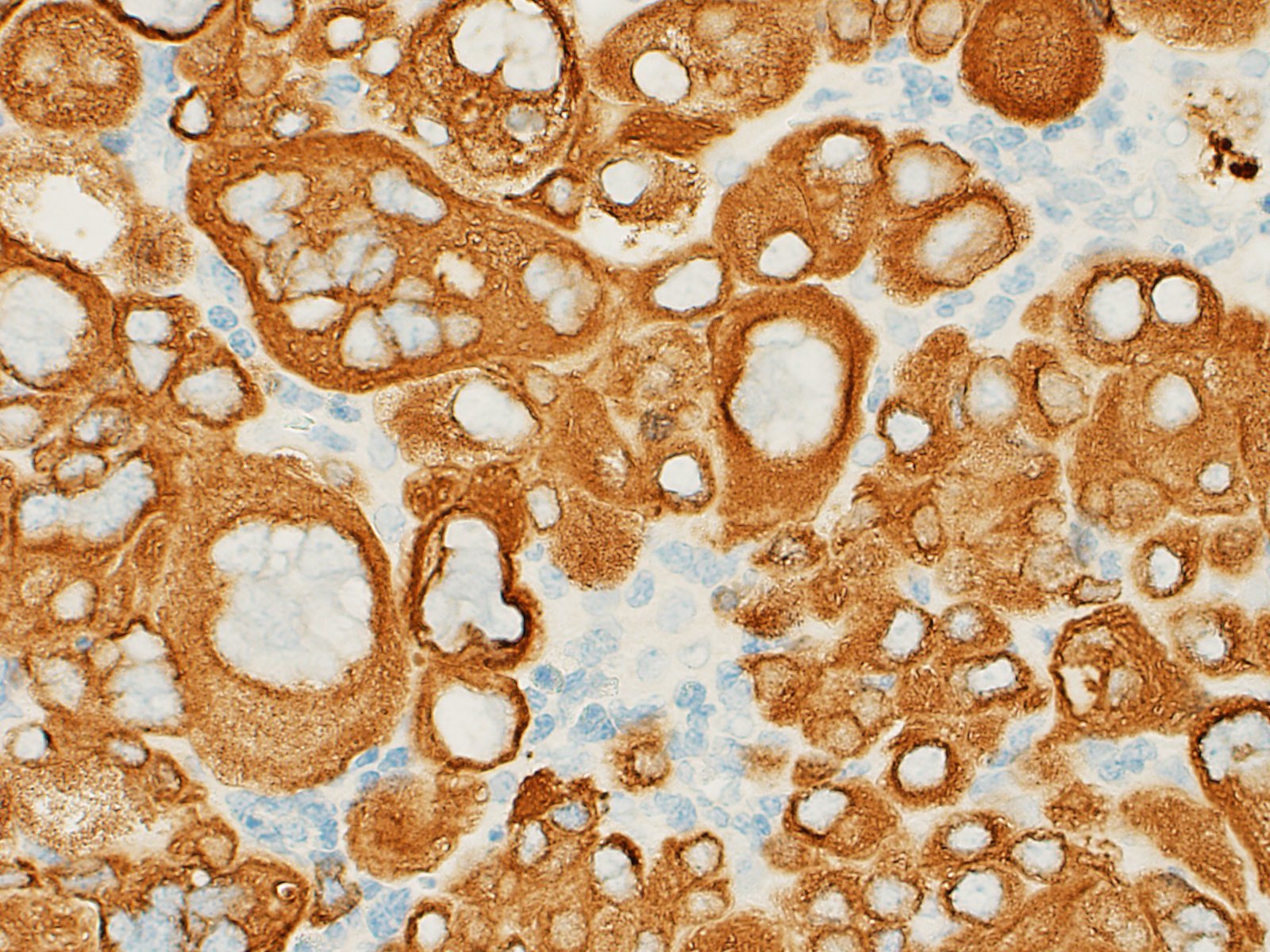
Center, which demonstrated patchy but strong immunopositivity of the
syncytiotrophoblastic population for hCG, and a weaker staining pattern for HPL
and weak to absent staining of cytotrophoblasts for both. As expected, both
populations stained strongly positive for cytokeratin, but this is not useful
in differentiating any of the three trophoblastic tumors. Based on this
findings and the histologic appearance of the tumor, we are in agreement with
the contributor that this tumor is best classified as a choriocarcinoma.
The moderator mentioned that based on the thickness of the uterus, the endometrium is likely hyperplastic (a change of little significance in this particular slide).
References:
1. Braga A, Mora P de Melko AC, Noguieria-Rodrigues A, Amim Junior J, Resenda-Filhon J, Seckl MJ. Challenges in the diagnosis and treatment of gestational trophoblastic neoplasia worldwide. World J Clin Oncol 2019; 10(2):28-37.
2 Chu D, Shih IM, Knezevich M, Sheth S: Uterine epithelioid trophoblastic tumor in an African green monkey (Chlorocebus aethiops sabaeus). J Am Assoc Lab Anim Sci 2007:46(2):92-96.
3 Cline JM, Wood CE, Vidal JD, Tarara RP, Buse E, Weinbauer GF, et al.: Selected Background Findings and Interpretation of Common Lesions in the Female Reproductive System in Macaques. Toxicologic Pathology 2008:36(7):142S-163S.
4 Cooper TK, Shih IM, Gabrielson KL: Uterine epithelioid trophoblastic tumour in a red-tailed guenon (Cercopithecus ascanius). J Comp Pathol 2005:133(2-3):218-222.
5. Elmore SA, Carreira V, Labriola CS et a. Proceedings of the 2018 National Toxicology Program Satellite Symposium. Toxicol Pathol 2018; 46(8)865-897.
6. Farman CA, Benirschke K, Horner M, Lappin P: Ovarian choriocarcinoma in a rhesus monkey associated with elevated serum chorionic gonadotropin levels. Vet Pathol 2005:42(2):226-229.
7. Giusti AM, Terron A, Belluco S, Scanziani E, Carcangiu ML: Ovarian epithelioid trophoblastic tumor in a cynomolgus monkey. Vet Pathol 2005:42(2):223-226.
8.. Lucas R, Cunha TM, Santos FB. Placental site trophoblastic tumor: a case report and review of the literature. Obstet Gyn Radiol 2015; 9(4):14-22
9.. Luiza JW, Taylor SE, Gao FF, Edwards RP: Placental site trophoblastic tumor: Immunohistochemistry algorithm key to diagnosis and review of literature. Gynecol Oncol Case Rep 2014:7:13-15.
10. Marbaix E, Defrere S, Duc KH, Lousse JC, Dehoux JP: Non-gestational malignant placental site trophoblastic tumor of the ovary in a 4-year-old rhesus monkey. Vet Pathol 2008:45(3):375-378.
11. Shih IM, Kurman RJ. Epithelioid trophoblastic tumor: a neoplasm distinct from choriocarcinoma and placental site trophoblastic tumor simulating carcinoma. Am J Surg Pathol 1998; 22(11):1393-1403.
12. Silverberg SG, Kurman RJ. Gestational trophoblastic disease. In: Atlas of Tumor Pathology, Vol 3. Tumors of the Uterine Corpus and Gestational Trophoblastic Disease. ARP Press, Washington D.C. 1991,
13. Stevens FT,
Katzorke N, Tempfer C, Kreimer U, Bizjak GI, Fleisch MC, et al.: Gestational
Trophoblastic Disorders: An Update in 2015. Geburtshilfe Frauenheilkd
2015:75(10):1043-1050.
14. Sunish Mohanan JC, Nancy Kock, Hermina Borgerink, Justin Vidal, Ross Tarara, J. Mark Cline. : Uterine Trophoblastic Tumors in Cynomolgus Macaques (Macaca fascicularis). Poster: Wake Forest School of Medicine.
15. Toyosawa K, Okimoto K, Koujitani T, Kikawa E: Choriocarcinoma and teratoma in the ovary of a cynomolgus monkey. Vet Pathol 2000:37(2):186-188.
16. Williams Gynecology, Third Edition: McGraw-Hill Education, 2016. https://accessmedicine.mhmedical.com/book.aspx?bookID=1758