Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 1
August 22nd, 2018
CASE II: BC/EP/NTP (JPC 4023574).
Signalment: 2-year-old female, Wistar-Han, Rattus norvegicus, rat
History: Tissue from a rat used in used in a National Toxicology Program (NTP) chronic toxicity/carcinogenesis study submitted for peer review. Clinically, the rat was reported as thin, lethargic, breathing abnormally and having a ruffled hair coat.
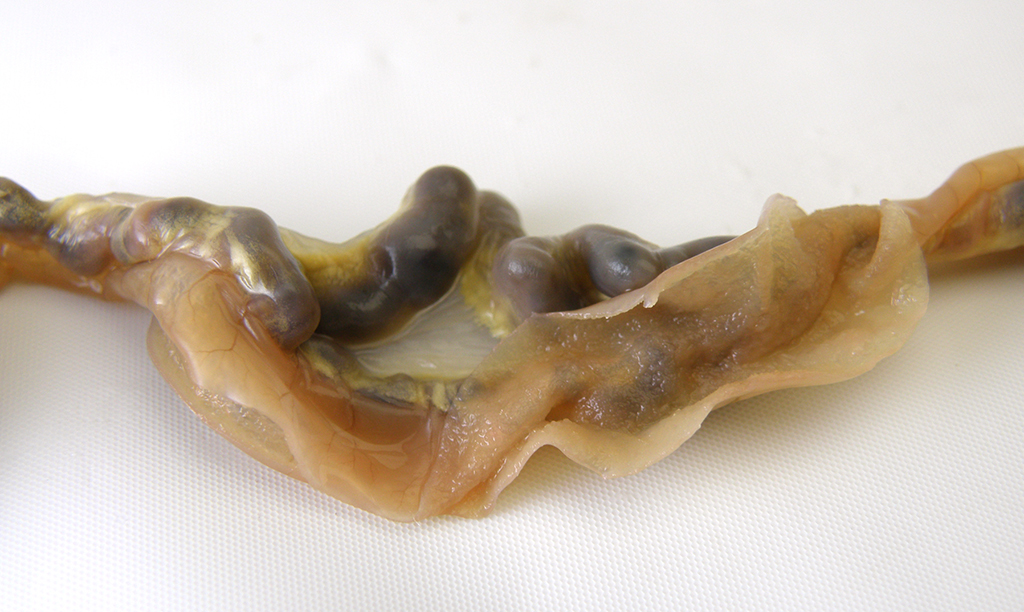
Gross Pathology: The individual animal necropsy record stated that multiple mesenteric blood vessels were enlarged (up to 10 x 5 x 5 mm). Other findings included bilateral granular appearing renal cortices and a 2 x 15 x 15 mm mass in the left inguinal mammary gland. The right ovary contained a 10mm diameter clear, fluid-filled cyst.
Laboratory results: None.
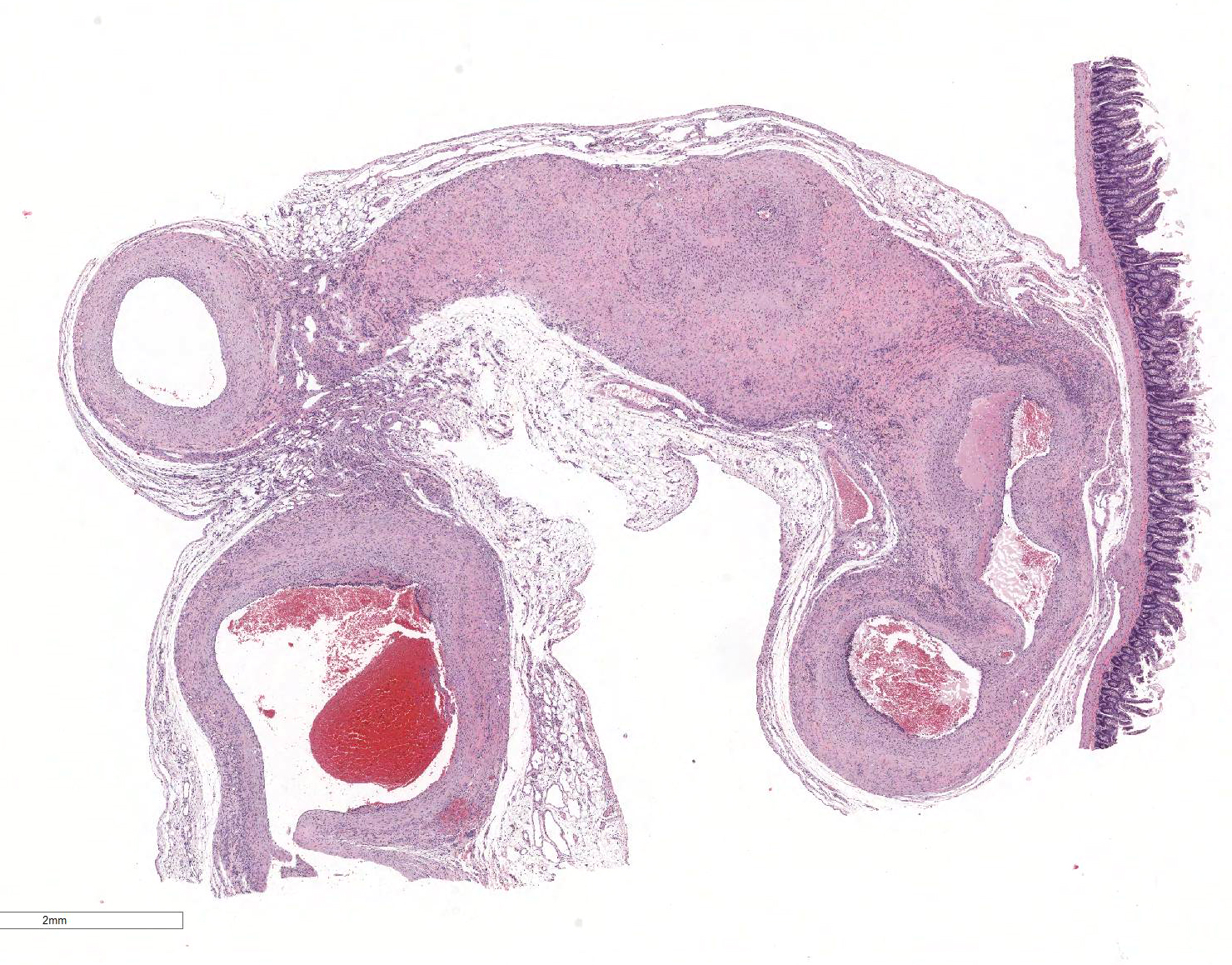
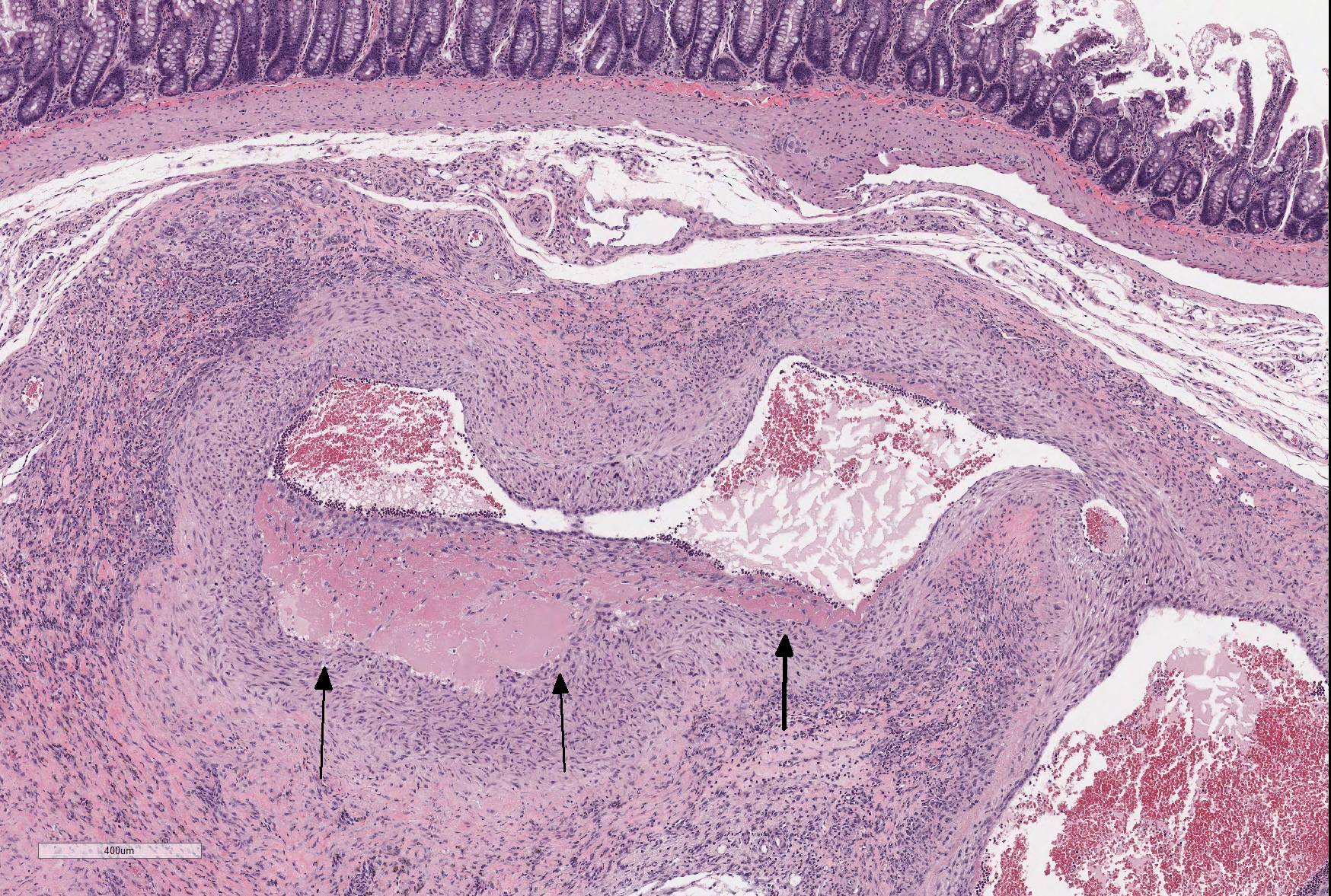
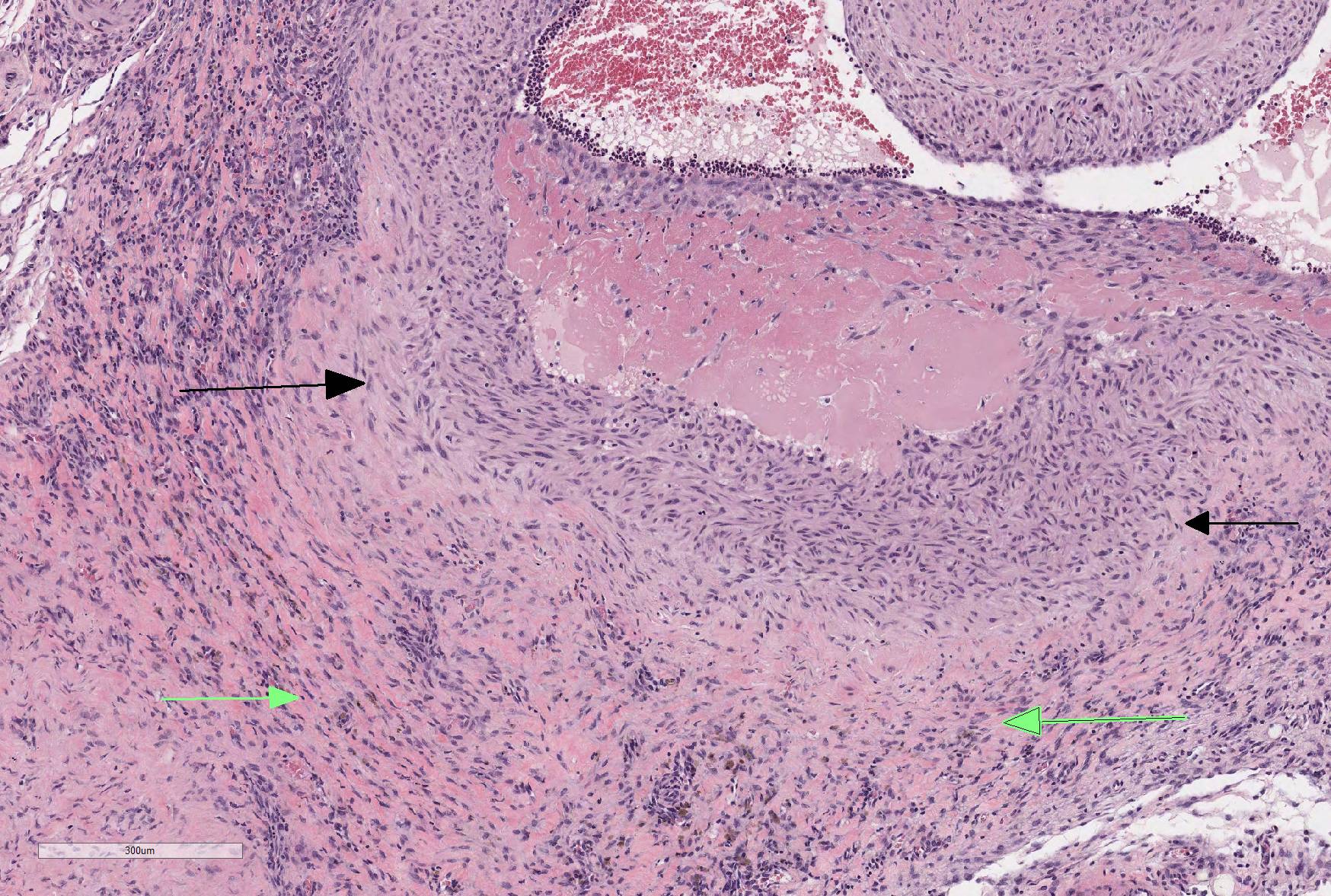
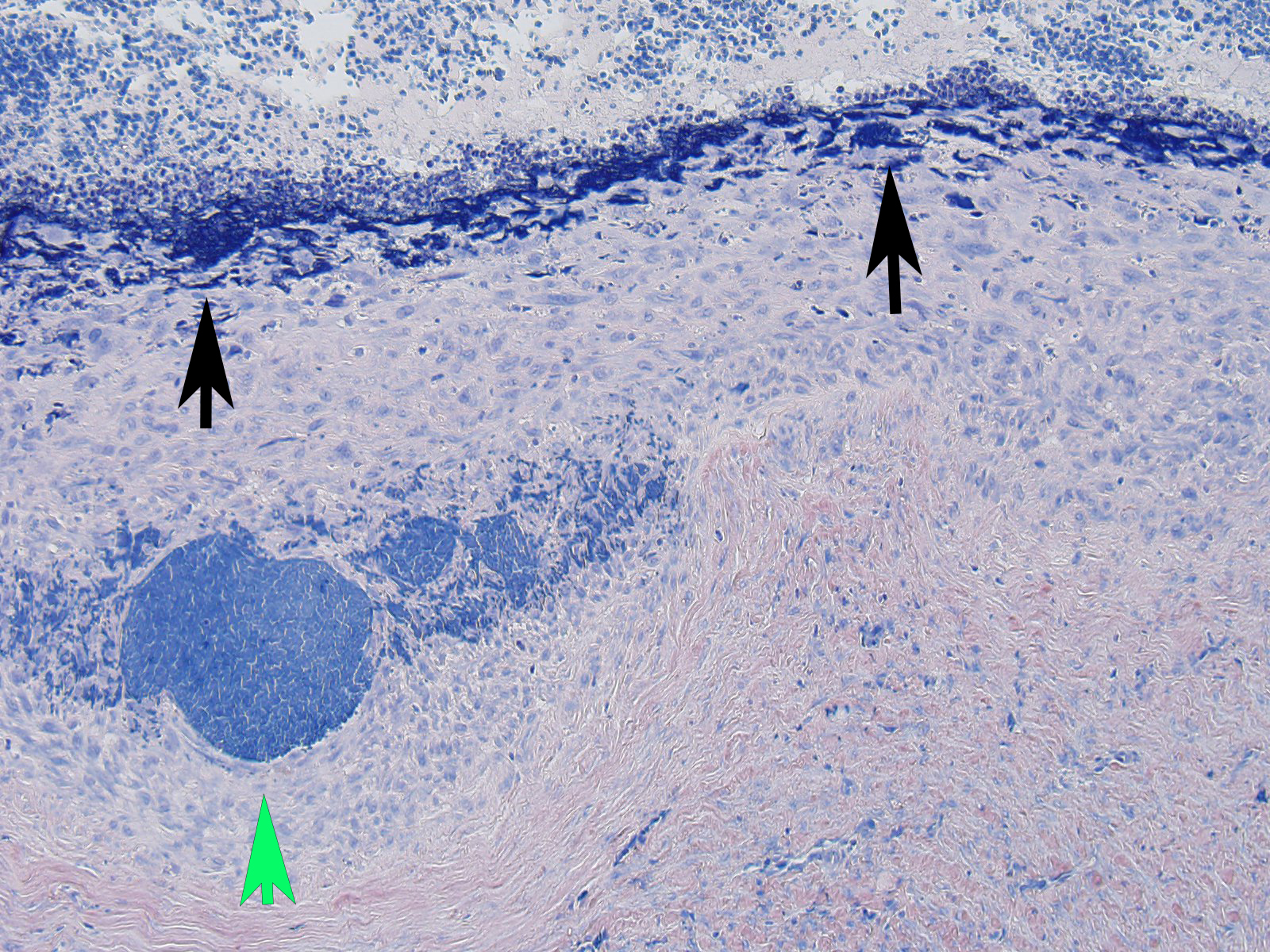
Microscopic Description: The section is of small intestine (ileum) and attached mesentery. The mesenteric artery is dilated and has a thickened wall surrounded by a varying amount of neovascularized connective tissue (granulation tissue). There is margination of neutrophils with focal necrosis of endothelium, inflammatory cell infiltrates, and variable amounts of fibrinoid material in some segments/sections. The tunica media is thickened by fibromuscular tissue and focal accumulations of lymphocytes and plasma cells are present at the periphery. Marked medial hypertrophy and thrombosis are present in some sections.
Contributorâs Morphologic Diagnoses: Artery, mesenteric â arteritis and periarteritis, chronic-active and proliferative, severe with multifocal necrosis and thrombosis.
Contributorâs Comment: The slide presents a typical case of spontaneous periarteritis (polyarteritis) nodosa of rats. Polyarteritis nodosa (PN) is a multisystemic, transmural necrotizing vasculitis of small or medium-sized muscular arteries.11 PN is the most conspicuous inflammatory vascular lesion of rats; the incidence varies among different strains. Incidences have been reported in the August (45.4% in males and 43.0% in females), Fischer 344 (1.8% in male and 0.9% in females), Long-Evans (4.5% in males and 2.6% in females) and in Wistar (9.1% in males and 2.6% in females)7 and spontaneously hypertensive rats (SHR) (76.1% in males and 10.3% in females).11
In the well-studied SHR rat, PN mainly affects the spermatic artery, mesenteric arteries, kidney, adrenal, and pancreas and is seen less frequently in the tongue, paratracheal tissue, salivary gland, liver, esophagus, thyroid, spleen, urinary bladder and intestine.12 Suzuki, et al. reported that PN was never seen in the lung, brain and aorta.12 Though PN lesions develop later in the mesenteric arteries than in testicular arterioles, the lesions of PN progress much faster in mesenteric arteries.10 Lesions of PN develop earlier and have a higher incidence in stroke-prone spontaneously hypertensive rats (SHRSP) than in stroke resistant (SHRSR) SHRs.12 PN lesions are often found at bifurcations of medium-sized arteries, small arteries and arterioles.12
A number of chemicals have been reported to produce vascular lesions.5,6,9,10,12 Fenoldopam mesylate (FM), a selective post-junctional dopaminergic (DA1) vasodilator, causes lesions of large caliber splanchnic arteries (100-180 micrometers) in the rat characterized by necrosis of medial smooth muscle cells and hemorrhage.5 FM does not induce lesions in other vascular beds of the rat, or in dogs or monkeys.5 Dopamine is an alpha- and beta-adrenoreceptor and dopaminergic receptor agonist.5 Dopamine, like FM, causes hemorrhagic lesions of large caliber splanchnic arteries in the rat, as well as fibrinoid necrosis of small caliber arteries (less than 100 micrometer) of the splanchnic, cerebral, coronary and renal vascular beds.5 Co-exposure to dopamine and an alpha adrenoreceptor antagonist (phenoxybenzamine) blocked fibrinoid necrosis of distal branches of the mesenteric arcade but increased severity of hemorrhagic lesions of larger arteries.5 Dopamine alone did not induce medial necrosis and hemorrhage of the rat pancreatic artery but phenoxybenzamine pretreatment resulted in characteristic lesions of the type induced by FM at this site.5 No rats exposed to dopamine and a combination of phenoxybenzamine and a DA1 receptor antagonist developed arterial lesions of any kind.6 The arterial lesion produced by FM and dopamine was not the result of alpha-adrenoreceptor-mediated vasoconstriction because phenoxybenzamine pretreatment provided no protection.5
Phosphodiesterases (PDE) are a family of enzymes responsible for the metabolism of the intracellular second messengers cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). Phosphodiesterase-4 (PDE4) is the major cAMP metabolizing enzyme in inflammatory and immune cells and makes a significant contribution to cAMP metabolism in airway smooth muscle cells.6 PDE4 inhibition produces bronchodilation, reduces vascular leakage in airways and modulates airway inflammation.6 Some PDE4 inhibitors produce arterial lesions in the mesentery of the rat; however, the lesions appear to begin in the mesenteric fat rather than in the arterial wall.6
Theophylline, an alkyloid and non-specific phosphodiesterase inhibitor found in cocoa and tea, is used medicinally as a bronchodilator and has been reported to induce mesenteric arteritis in male F344/N rats exposed chronically for 2 years at 75mg/kg and in both sexes in acute (16 day) studies at 40mg/kg given once daily.7 The mesenteric and pancreatic arteries of rats are particularly sensitive to excessive vasodilator activity.7
JPC Diagnosis: Mesenteric artery: Arteritis, necrotizing and proliferative, multifocal, chronic-active, marked, with fibrinoid necrosis, mural smooth muscle hyperplasia and marked medial and adventitial fibrosis.
Conference Comment: Lesions consistent with polyarteritis nodosa are not exclusive to the rat but have been documented in a wide range of species including mice, sheep, raccoons, pigs, dogs, non-human primates, and man.
While PN in many instances and in many species is idiopathic, a number of plausible theories have been purported to explain its cause. In humans, PN is a disease primarily of young adults which affects the small-or medium-sized muscular arteries typically involving renal vessels but sparing the pulmonary circulation.2 Lesions are very similar to those seen in this case, down to the often segmental, non-circumferential areas of fibrinoid necrosis, with a predilection for vascular branching points.1 Approximately 30% of PN cases in humans or the result of infection with hepatitis B, as antigen-antibody complex is within affected vessels off and are immunopositive for hepatitis B antigen. 2 The lesions of PN blue foxes infected with Encephalitozoon cuniculi 7 have also been suggested as a potential infectious cause for the classic Type III hypersensitivity lesions in this condition.
A number of related theories abound regarding the development of PN in rats administered a variety of structurally unrelated vasodilators listed in the contributorâs comments. The reduction in mural tension of splanchnic vessels in the face of the positive inotropy caused by these drugs may result in increased shear forces to endothelium and damage to the underlying tunica media, as well as the possibility of interference with the diffusion of essential nutrientâs into the wall affected arteries increasing there are susceptibility to injury.9 In affected rats, mesenteric and pancreatic arteries are particularly sensitive to these types of drugs, which may be the result of the lack of supporting tissue for the splanchnic vasculature.9 It has also been suggested that arterial necrosis develops due to alternating dilation and vasoconstriction of these muscles, which may help to explain the prevalence of this condition in spontaneously hypertensive rats.
Phosphodiesterase-inhibiting drugs, which have positive inotropic effects on the heart, have also been shown to produce distinctive arterial changes of the extramural coronary arteries with subsequent myocardial necrosis and atrial and endocardial hemorrhage in the dogs as well.3 (See WSC 2009-2010, Conference 2, case III). At 7 days of treatment or less histologic changes include medial necrosis and hemorrhage, and more chronic treatment protocols are required to achieve the proliferative changes of the intima and adventitia which characterize PN. Interestingly, associated clinical signs of fever, weight loss, cervical neck pain, and neutrophilic leukocytosis are seen in idiopathic polyarteritis of beagles (âbeagle pain syndromeâ), but not with administration of vasoactive compounds.2
An interesting view on the development of PN in rats following administration of a phosphodiesterase type for inhibitor was put forth an article by Mecklenburg et al.6 In a timed dosage study, rats administered a phosphodiesterase inhibitor developed a mesenteritis as early as 3 days after the initiation of drug treatment, which was characterized by macrophage infiltration, fibroblast proliferation, neovascularization, and atrophy and loss of adipocytes (similar to that seen in this case). A segmental necrotizing panarteritis was subsequently seen but only after 3 or 4 weeks of treatment, and only in a limited subset of individuals. The authors of this study5 postulate that phosphodiesterase inhibitors do not cause a primary vasculitis in rats but the predominant toxic effects are seen in the mesentery, and that cytokines, growth factors, and other mediated results of inflammation in the adjacent mesentery may result in vascular lesions.
A recent article has been published on spontaneous arteritis and its comparison with drug-induced arteritis in Gottingen minipigs.3 Arteritis is a common background finding in Gottingen minipigs used in preclinical safety studies, and is most often seen in cardiac vessels, vagina, oviduct, rectum, epididymis, spinal cord, pancreas, urinary bladder, kidney, and stomach. It is of unknown etiology, and may be seen in association with thrombocytopenic purpura syndrome, an often fatal disease in this breed. The article discusses the relative resistance of minipigs to drug-induced arteritis as compared to the rats, dogs, and monkeys, and the absence of lesions associated with admonition of endothelin receptor antagonists and phosphodiesterase inhibitors.3
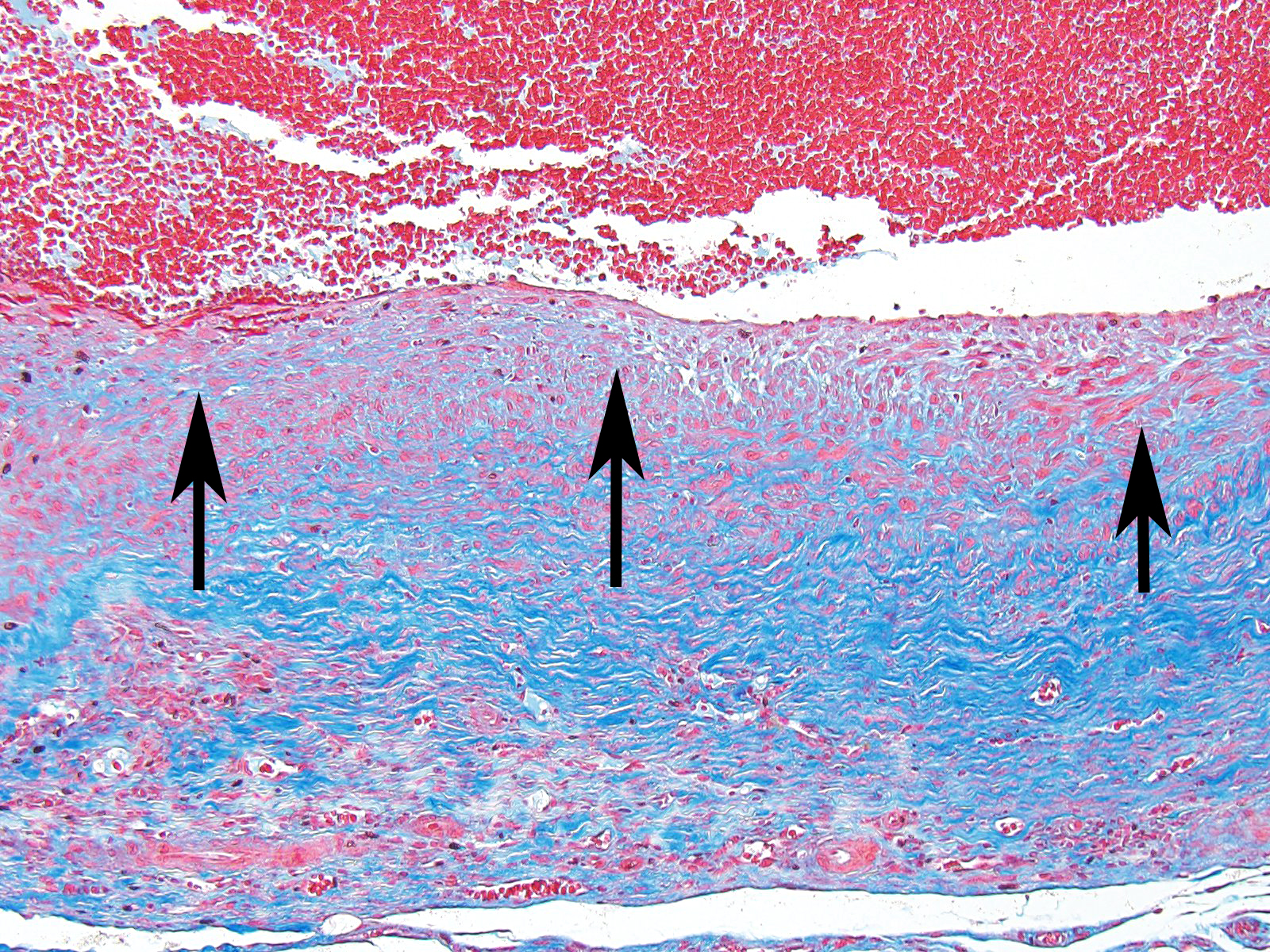
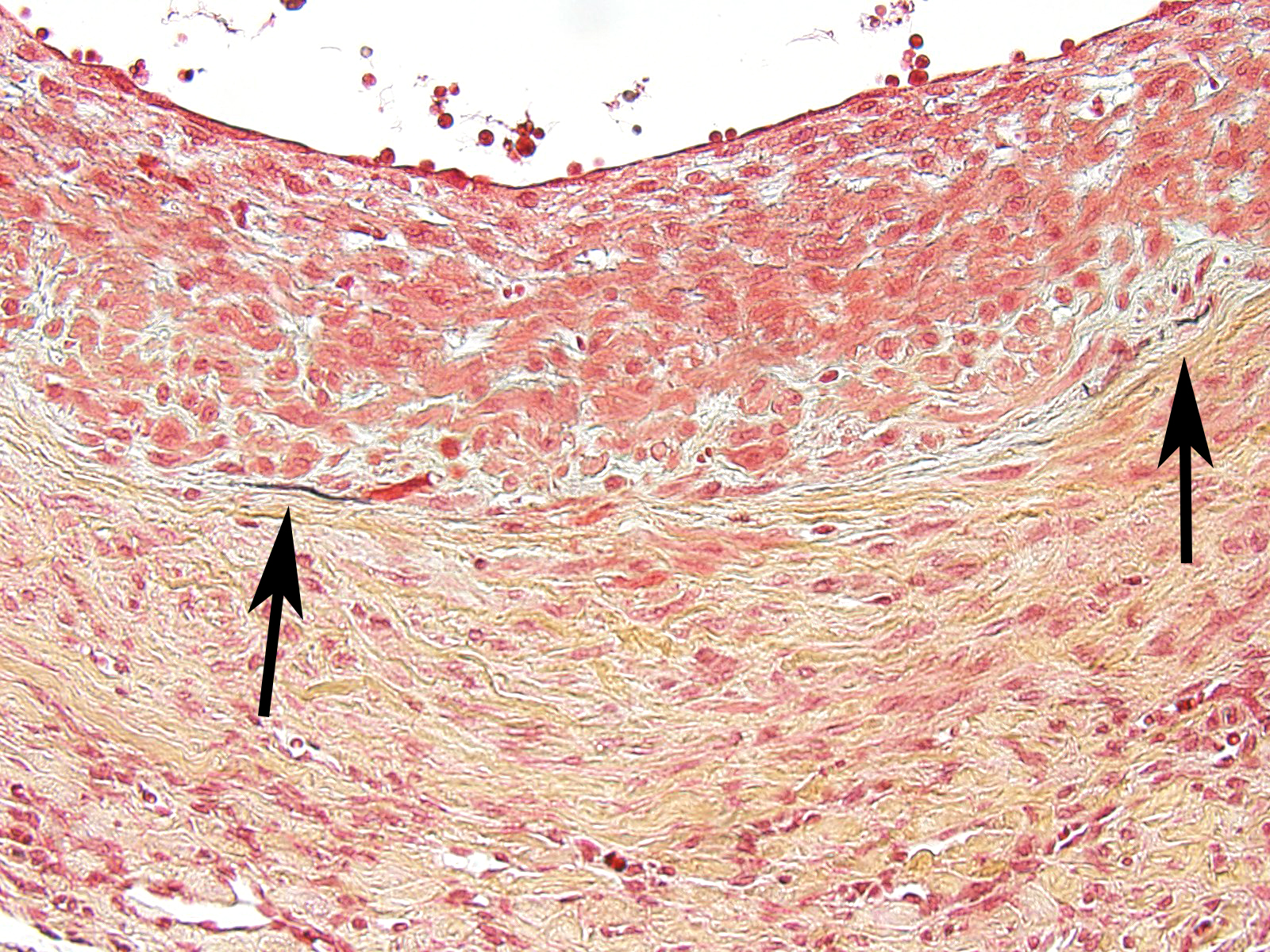
The moderator started the discussion by reviewing a recent article by Bacares1, detailing an excellent method for the histologic examination of mesenteric artery of the rat. A review of special stains for this case (Massonâs trichrome and Movat pentachrome) demonstrated a number of important features including the loss of the internal elastic lamina, discontinuity of the external elastic lamina, and the marked fibrosis of the tunica media and adventitia.
Contributing Institution:
Experimental Pathology Laboratories, Inc., www.epl-inc.com
References:
- Bacares, MEP. Sampling the rat mesenteric artery: a simple technique. Toxicol Pathol 2016; 44(8): 1166-1169.
- Clemo FAS, Evering W, Snyder PW, Albassam MA: Differentiating spontaneous from drug-induced vascular injury in the dog. Toxicol Pathol 31(Suppl.):25-31, 2003.
- Dincer AD, Piccicuo V, Walker UJ, Mahl A, McKeag S: Spontaneous and drug-induced arteritis/polyarteritis in the Gottingen minipig â review. Toxicol Pathol, 2018; 46(2):121-130.
- FerrerasMC, Benavides J, Fuertes M, et al. Pathological features of systemic necrotizing vasculitis (polyarteritis nodosa) in sheep. J Comp Pathol. 2013;149:74-81.
- Kerns WD, Arena E, Macia RA, Bugelski PJ, Matthews WD, Morgan GD: Pathogenesis of arterial lesions induced by dopaminergic compounds in the rat. Toxicol Pathol 1989; 17:203-213.
- Mecklenburg L, Heuser A, Juengling T, Kohler M, Foell, R, Ockert D, Tuch K, Bode G: Mesenteritis precedes vasculitis in the rat mesentery after subacute administration of a Phosphodiesterase type 4 inhibitor. Toxicol Ltt 2006; 163:54-64.
- Mitsumori K: Blood and lymphatic vessels. In: Boorman GA, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF. Pathology of the Fischer Rat. San Diego, CA: Academic Press; 1990:475-477.
- Nordstoga K, Westbye K. Polyarteritis nodosa associated with nosematosis in blue foxes. Acta Pathol Microbiol Scand A 1976; 84(3):291-296
- Nyska A, Herbert RA, Chan PC, Haseman JK, Hailey JR: Theophylline-induced mesenteric periarteritis in F344/N rats. Arch Toxicol 1998; 72: 731-737.
- Saito N, Kawamura H: The incidence and development of periarteritis nodosa in testicular arterioles and mesenteric arteries of spontaneously hypertensive rats. Hypertens Res 1999; 22:105-112.
- Schoen FJ, Cotran RS: Blood vessels. In: Cotran RS, Kumar V, Collins T, eds. Robbins Pathologic Basis of Disease. 6th Philadelphia, PA: W.B. Saunders Company; 1999: 520-521.
- Suzuki T, Oboshi S, Sato R: Periarteritis nodosa in spontaneously hypertensive rats â incidence and distribution. Act Path Jap 1979; 29:697-703.
- Wessels 1, StrugnellB,, Woodger N, Peat M, La Rocca SA, Dastjerdi A. Systemic necrotizing polyarteritis in three weaned lambs from one flock. J Vet Diagn Invest 2017; 29(5) 733â737