CASE 4: N1929202 (4153152-00)
Signalment:
Adult, intact male, domestic shorthaired cat, Felis catus, Feline.
History:
Three cats (two intact males and one intact female) were found dead in the same area and were suspected to have been poisoned. The cats were submitted for postmortem examination.
Gross Pathology:
All cats were in good body condition with adequate fat stores. Granular, dark red-black blood was detected in the stomach of each cat. One cat had numerous pinpoint bleeding gastric erosions/ulcers. A different cat had multifocal erosions in the urinary bladder erosion and the lumen contained a coagulum of fibrin and necrotic debris. The kidneys of all three cats were grossly unremarkable.
Laboratory results:
Gas Chromatography Mass Spectrometry (GC-MS) was performed on fresh kidney tissue and ethylene glycol was detected above a level of 50 ppm.
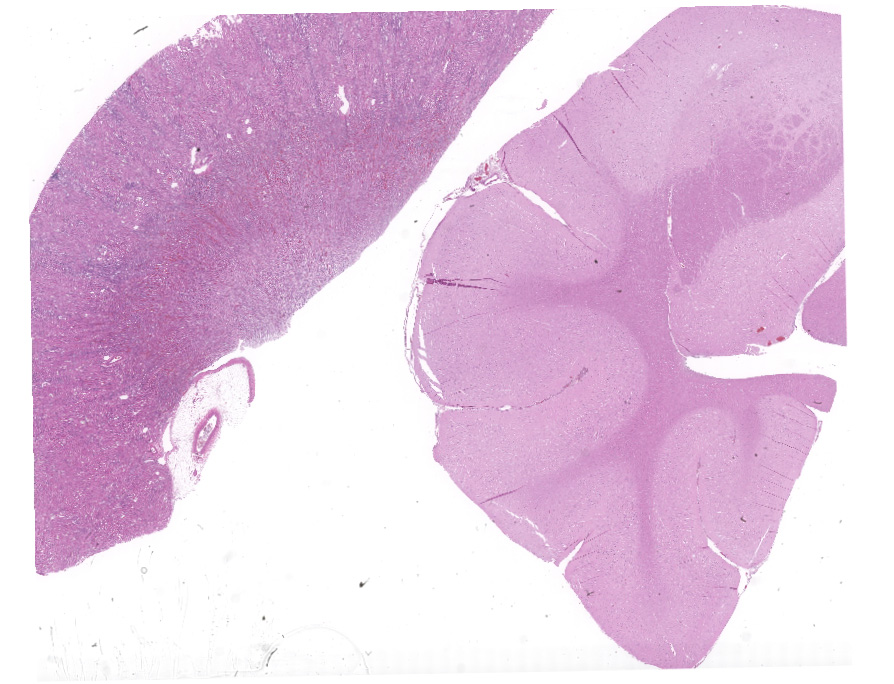
Microscopic description:
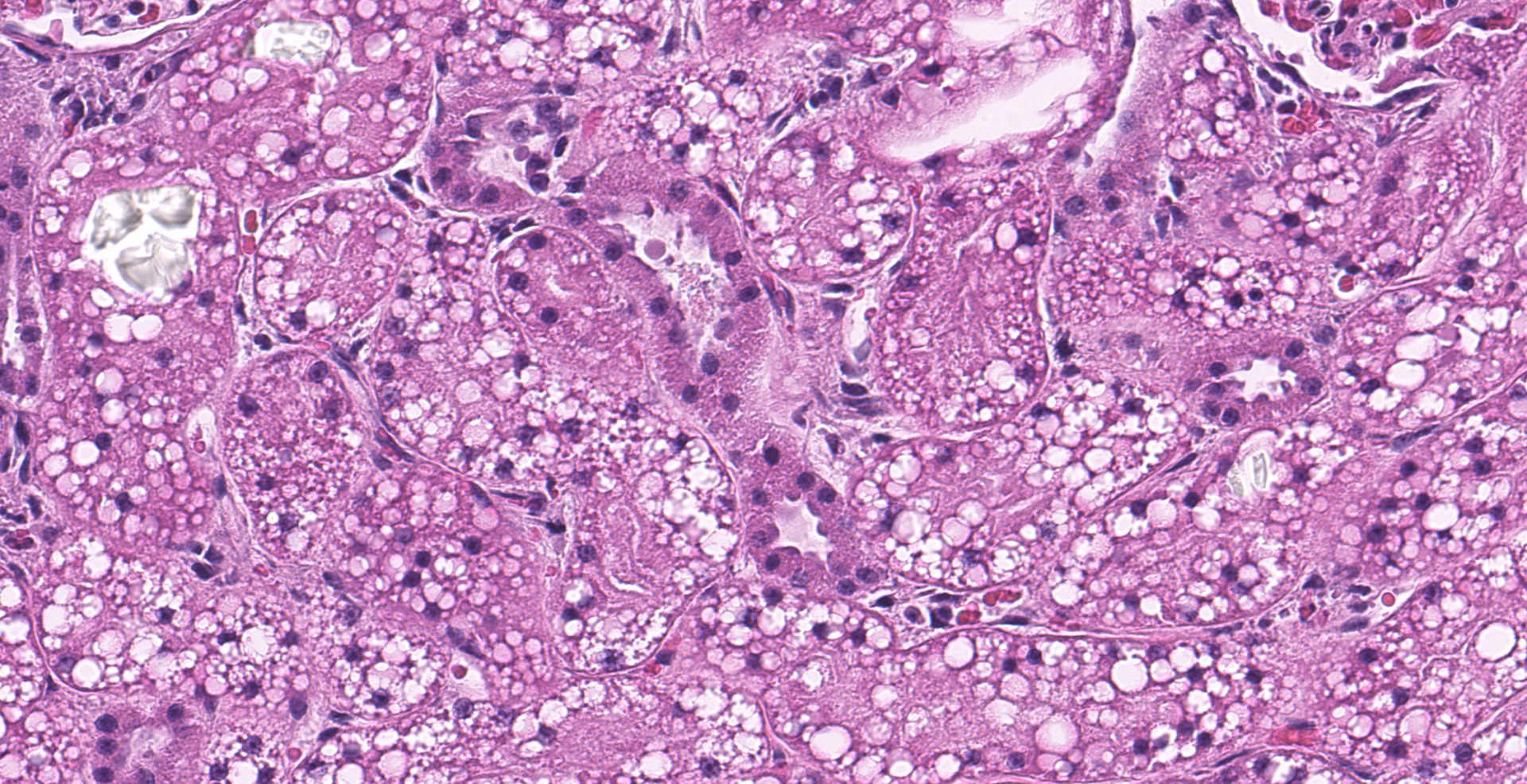
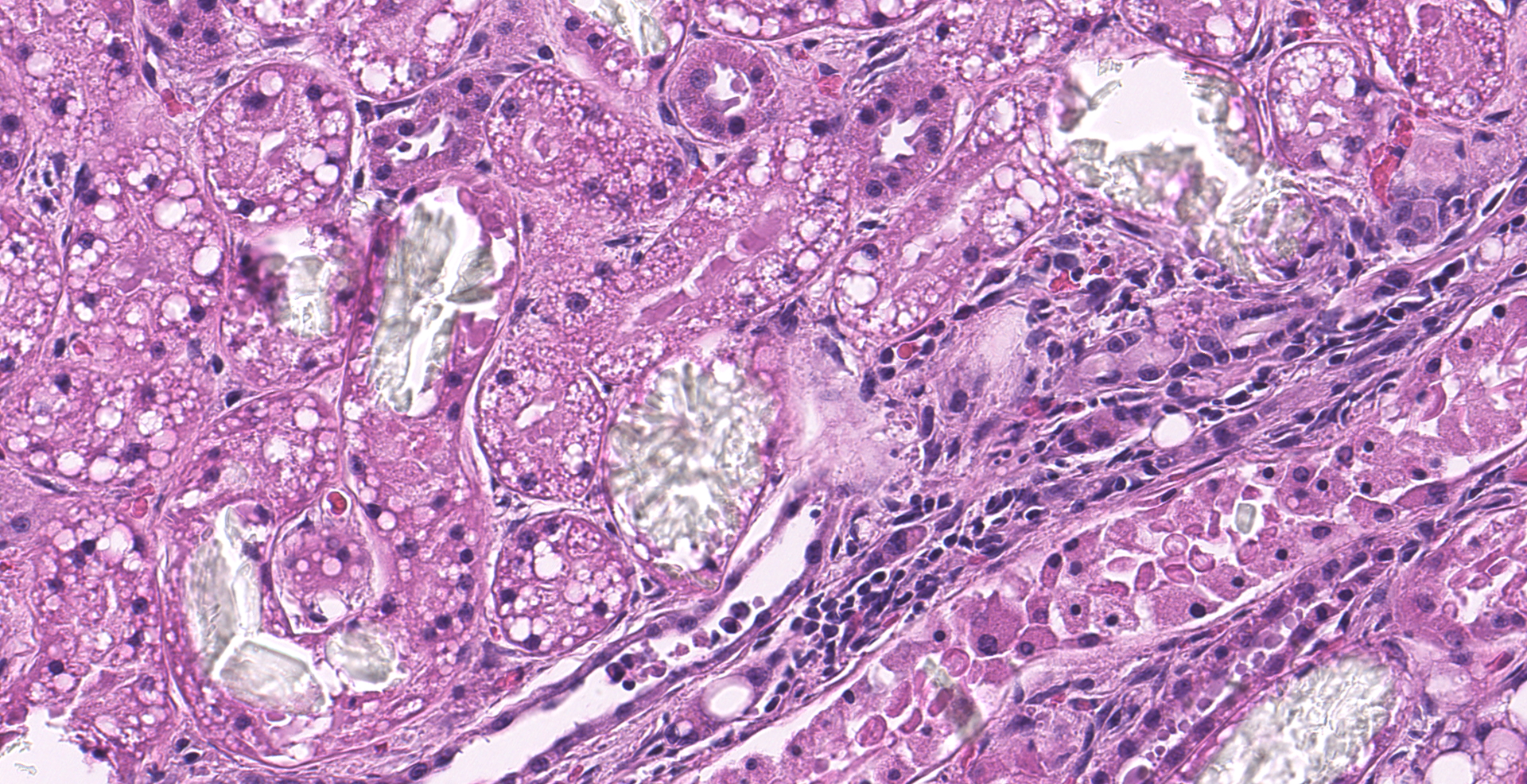
Multifocally, numerous renal cortical, and to a lesser extent medullary, tubules are distorted and expanded by radiating colorless crystalline material that is birefringent under polarized light (calcium oxalate crystals). The crystals often fill tubular lumina and disrupt tubular epithelia. Affected tubular epithelial cells are attenuated and degenerate or necrotic with shrunken with hypereosinophilic cytoplasm and pyknotic nuclei. Necrotic cells are occasionally sloughed into the lumen, admixed with cellular debris and eosinophilic fluid. There are few granular and cellular casts and renal epithelia are swollen and contain variably discrete lipid vacuoles (renal lipidosis).
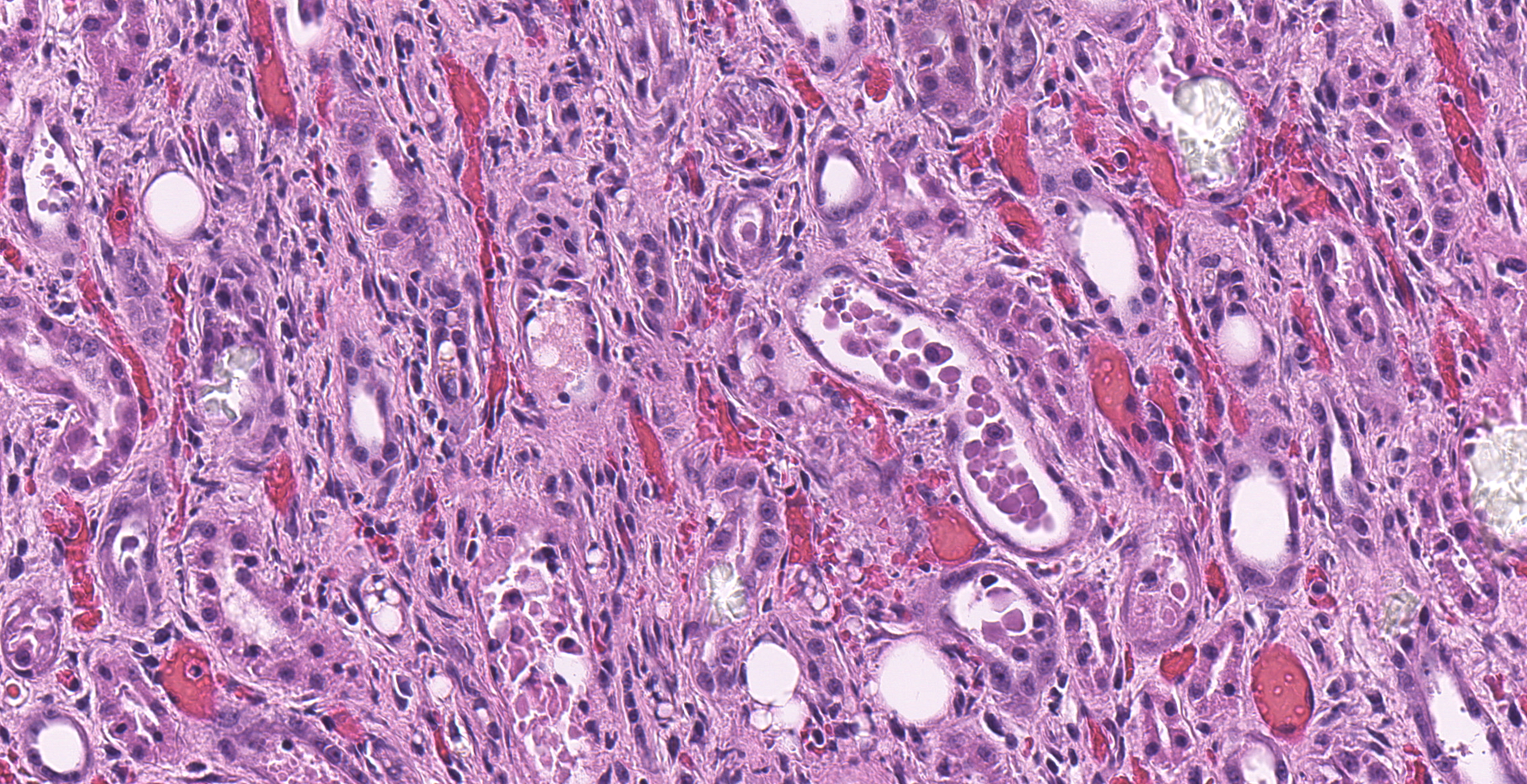
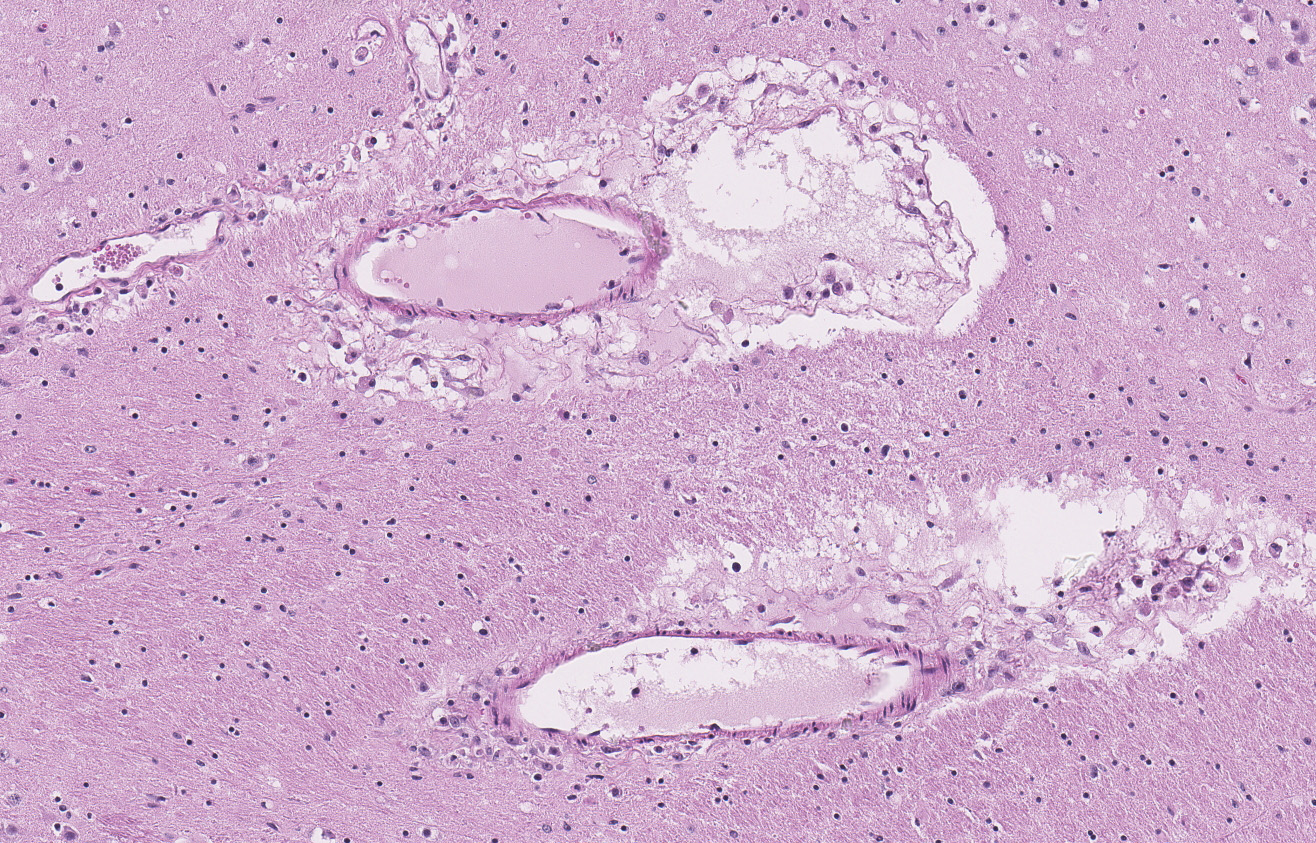
Within the cerebral parenchyma and leptomeninges, birefringent calcium-oxalate crystals multifocally expand the perivascular space, disrupt vessel walls, or rarely, protrude into the vascular lumen. Disrupted vessel walls are replaced by crystals, karyorrhectic debris, and neutrophils. Neutrophils extend into Virchow-Robins space along with small eosinophilic droplets (edema). In less severely affected blood vessels, endothelia are hypertrophied. There is a mild generalized increase in glial cells, predominately astrocytes.
Contributor's morphologic diagnosis:
Kidney, cat: severe acute nephropathy characterized by numerous intratubular crystals, multifocal acute tubular degeneration and necrosis
Brain, cat: numerous perivascular crystals with perivascular and leptomeningeal neutrophilic and lymphohistiocytic infiltrates
Contributor's comment:
Voluntary ingestion of sweet-tasting antifreeze by cats and dogs is the most common presentation of ethylene glycol (EG) toxicosis. Temperate and cold climates during the Fall and Spring have the highest incidence of cases, coinciding with the highest use of this product.2 Ethylene glycol and its metabolites result in three overlapping stages of disease: central nervous system depression and gastrointestinal upset, metabolic acidosis, and finally acute tubular injury and renal failure.14 EG itself is relatively non-toxic and systemic effects are due to ethylene glycol metabolites.7,8 Death can occur predominantly from development of severe metabolic acidosis and acute renal failure. Hyperkalemia from renal failure can induce cardiac arrhythmias and metabolic acidosis can lead to central nervous system depression/seizures/coma and pulmonary edema causing respiratory distress and dysfunction.14 Lethargy, anorexia, dehydration, vomiting, diarrhea, uremic ulcers, and salivation are other common symptoms that can occur.14
The metabolism of EG involves a series of rate-limiting and non-rate-limiting steps in the liver and kidneys. Absorption through the digestive tract is rapid, and the majority of EG is excreted through the kidney without being metabolized. The remainder of EG is converted to glycolaldehyde by alcohol dehydrogenase (rate-limiting step), quickly oxidized to glycolic acid, and then oxidized again to glyoxylic acid (rate-limiting step).8,12,14 Metabolism ends with the production of several compounds, of which oxalate and lactic acid are the most clinically important. Oxalate's importance is discussed in the following paragraph. Lactic acid contributes to the development of metabolic acidosis. The other end metabolites are hippuric acid and carbon dioxide.14 Once EG has been completely metabolized, treatments aimed at inhibiting alcohol dehydrogenase will not be effective. Prognosis at this stage of disease if guarded to poor.14
Calcium binds to oxalate and forms crystals within renal tubular lumina and is taken up by tubular epithelium and secreted into the renal interstitium.1,2 With decreased renal function, crystals will accumulate at high concentrations in circulation and deposit in perivascular spaces within other tissues such as the brain. Glycolaldehyde and glyoxylic acid are classically postulated to be primary effectors, though there has been some debate about the role of EG metabolites in causing direct cellular injury.7,12 Within the past two decades, multiple studies suggest that deposition of calcium oxalate crystals may be a larger contributor to acute tubular injury and development of renal failure through various proposed direct and indirect cytotoxic mechanisms.7,10,11
Clinicopathologic findings include a titrational metabolic acidosis due to the production of acidic metabolites and lactic acid (resulting in a markedly increased anion gap) and severe serum hyperosmolality with an increased osmolal gap.14 The urine is isosthenuric, acidic, and contains variable numbers of calcium oxalate monohydrate crystals beginning 3-6 hours after ingestion of the toxin.9,13 Few numbers of monohydrate crystals are normally found in the urine, but high numbers should increase the suspicion of EG toxicosis.9 Additional biochemical findings are transient hyperglycemia, hypocalcemia, and hyperphosphatemia, which is initially caused by phosphate rust inhibitors in the antifreeze and then persists due to renal dysfunction.9,14
At postmortem examination, swollen edematous kidneys, pulmonary edema, and hemorrhagic gastroenteritis are common gross findings, although in many cases gross lesions are not observed.1,2 Histologically, numerous birefringent calcium oxalate crystals within the renal tubules are the most characteristic and almost pathognomonic finding in cases of EG toxicosis. Primary hyperoxaluria, a rare inherited condition in humans, cats, dogs (Tibetan spaniels, Shih Tzu), and Beefmaster cattle, or ingestion of oxalate containing plants or oxalate producing fungi (such as Aspergillus spp.) are other differentials in these cases.2
An interesting histologic finding in these cats was the detection of perivascular crystals in the brain. The literature postulates that development of central nervous system signs is most likely because of aldehyde and the development of metabolic acidosis rather than the presence of the crystals.2 Few human case reports highlight delayed neurological effects from cerebral edema and obvious histologic vascular injury suggested to be due to the presence of crystals.5,6 At this point in time to the author's knowledge, primary research specifically examining effects of crystal formation on vessels and development of neurological signs is limited. In these cats, neutrophilic infiltrates were sometimes observed in association with perivascular crystal deposition. Inflammation was not present in all cats and was variable between cerebral sections. Immunohistochemical staining for feline infectious peritonitis (FIP) was negative. In this case, the inflammatory reaction was suspected to be secondary to vascular damage due to crystal deposition rather than a separate primary process.
Contributing Institution:
University of Pennsylvania
School of Veterinary Medicine
Department of Pathobiology
https://www.vet.upenn.edu/research/academic-departments/pathobiology
JPC diagnosis:
1. Kidney, tubules: Degeneration and necrosis, diffuse, marked, with numerous intratubular oxalate crystals, and granular and protein casts.
2. Cerebrum, perivascular areas: Edema, multifocal, moderate with neutrophilic and lymphohistiocytic infiltrates, and oxalate crystals.
JPC comment:
The contributor provides an excellent review of this condition in animals. Recent research has investigated the use of stiripentol, an antiepileptic drug used to treat Dravet syndrome in humans, to reduce the formation of calcium oxalate crystals upon ethylene glycol ingestion. Stiripentol inhibits lactate dehydrogenase 5, the final step of hepatic oxalate formation. Sixteen Sprague Dawley rats received hydroxyproline and calcium in water for 16 days, with half receiving stiripentol daily. The experimental group had lower resultant urine oxalate, suggesting a potential future therapy.3
Interestingly, while ethylene glycol leads to the disease described in this case, (poly)ethylene glycol is a polymeric molecule with properties that warrant investigation as medical therapies. In vitro exposure of metastatic melanoma cells (human, A375 cells) to different molecular weight (poly)ethylene glycol polymers resulted in morphologic signs of toxicity. This is far from a clinical intervention to date but may lead to interesting therapies in the future.13
While in veterinary medicine, we typically think of ethylene glycol in the context of automobile antifreeze. But ethylene glycol is also integral to the production of numerous items, such as polyethylene terephthalate (PET) plastics, deicing fluid for aircraft operations, and as an inhibitor of clathrate hydrate formation in natural gas pipelines. With such diverse applications, many involving open-air release, ethylene glycol is a common environmental pollutant that is slow to chemically decompose. Bacteria have been leveraged to assist with the breakdown of numerous plastics, and an engineered strain of Pseudomonas putita has shown promise for degrading ethylene glycol to non-harmful byproducts. Specifically, P. putita KT2440 was engineered to overexpress glycoxylate carboligase (gcl) in combination with products of four other genes in close proximity (hyi, glxR, ttuD, pykF), resulting in efficient growth and utilization of ethylene glycol substrate. Research in this vein may assist in removing this toxic compound from the environment in the future.4
References:
1. Breshears MA, Confer AW. The urinary system. In: Zachary JF, ed. Pathological Basis of Veterinary Disease. 6th ed. Philadelphia, PA: Mosby Elsevier Inc.; 2017:675-679.
2. Ciancolo RE, Mohr FC. Urinary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier; 2016:425.
3. Dudal M, Huguet L, Perez J, et al. Stiripentol protects against calcium oxalate nephrolithiasis and ethylene glycol poisoning. J Clin Invest. 2019;129(6):2571-2577.
4. Franden MA, Jayakody L, Li WJ, et al. Engineering Pseudomonas putida KT2440 for efficient ethylene glycol utilization. Metabolic Engineering. 2018;48:197-207.
5. Froberg K, Dorion RP, McMartin KE. The Role of Calcium Oxalate Crystal Deposition in Cerebral Vessels During Ethylene Glycol Poisoning. Clinical Toxicology. 2006;44:3;315-318.
6. Garg D, Lim T, Irani M. A rare case of fatal stroke after ethylene glycol toxicity. BMJ Case Rep. 2015:1-3.
7. Guo C, McMartin KE. The cytotoxicity of oxalate, metabolite of ethylene glycol, is due to calcium oxalate monohydrate formation. Toxicology. 2005;208:3;347-355.
8. Jacobsen D., ØvrebØ S, Østborg J, Sejersted OM. Glycolate Causes the Acidosis in Ethylene Glycol Poisoning and is Effectively Removed by Hemodialysis. Acta Medica Scandinavica. 1984;216:409-416.
9. Latimer, KS editors. Duncan & Prasse's Veterinary Laboratory Medicine: Clinical Pathology; 5th Ed. Chichester, West Sussex, UK: Wiley-Blackwell; 2011.
10. McMartin K. Are calcium oxalate crystals involved in the mechanism of acute renal failure in ethylene glycol poisoning?. Clinical Toxicology. 2009;47:9;859-869.
11. Ozturk H, Cetinkaya A, Firat TS, Tekce BK, Duzcu SE, Ozturk H. Protective effect of pentoxifylline on oxidative renal cell injury associated with renal crystal formation in a hyperoxaluric rat model. Urolithiasis. 2019;47(5):415?424.
12. Podelski V, Johnson A, Wright S, Dela Rosa V, Zager RA. Ethylene glycol-mediated tubular injury: identification of critical metabolites and injury pathways. American Journal of Kidney Diseases. 2001;38:2;339-348.
13. Postic I, Sheardown H. Poly(ethylene glycol) induces cell toxicity in melanoma cells by producing a hyperosmotic extracellular medium. Journal of Biomaterials Applications. 2018;33(5):693-706.
14. Tiwari RM and Sinha M. Industrial Poisoning. In: Veterinary Toxicology. Oxford Book Company; Mehra Offset Press: Delhi, India, 2010;181-184.