WSC Conference 4, Case 1
Signalment:
1-month-old, Quarter Horse colt, horse (Equus caballus)
History:
This foal presented to the Equine Emergency Service acutely ill with a fever, tachypnea, diarrhea, and seizures. Laboratory data revealed elevated liver enzymes and bile acids, left shift neutropenia, elevated fibrinogen, hypoglycemia, hyperlactatemia, and electrolyte abnormalities (see details below under laboratory results). Abdominal ultrasound identified hepatomegaly. The foal was humanely euthanized due to poor prognosis.
Gross Pathology:
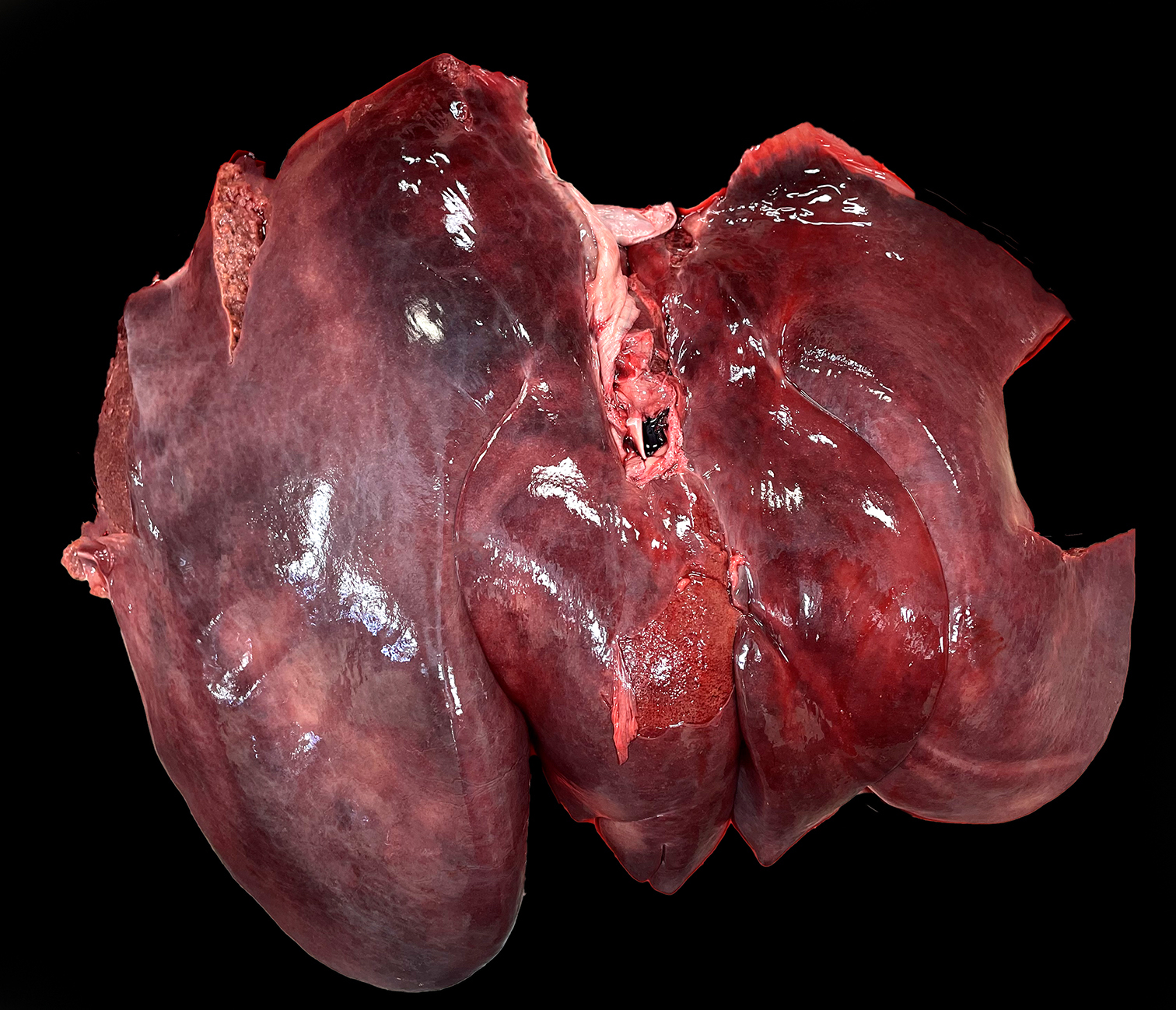
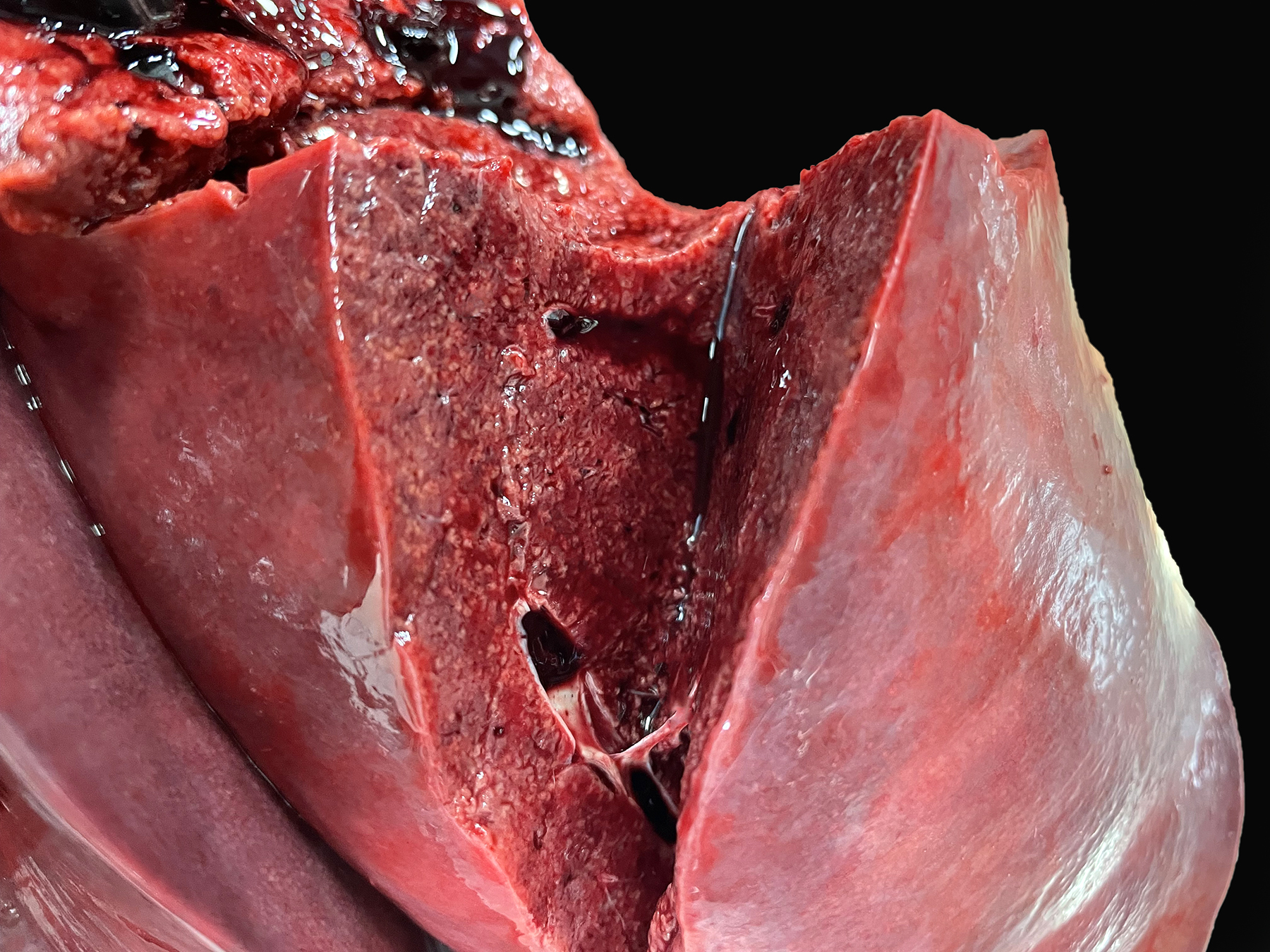
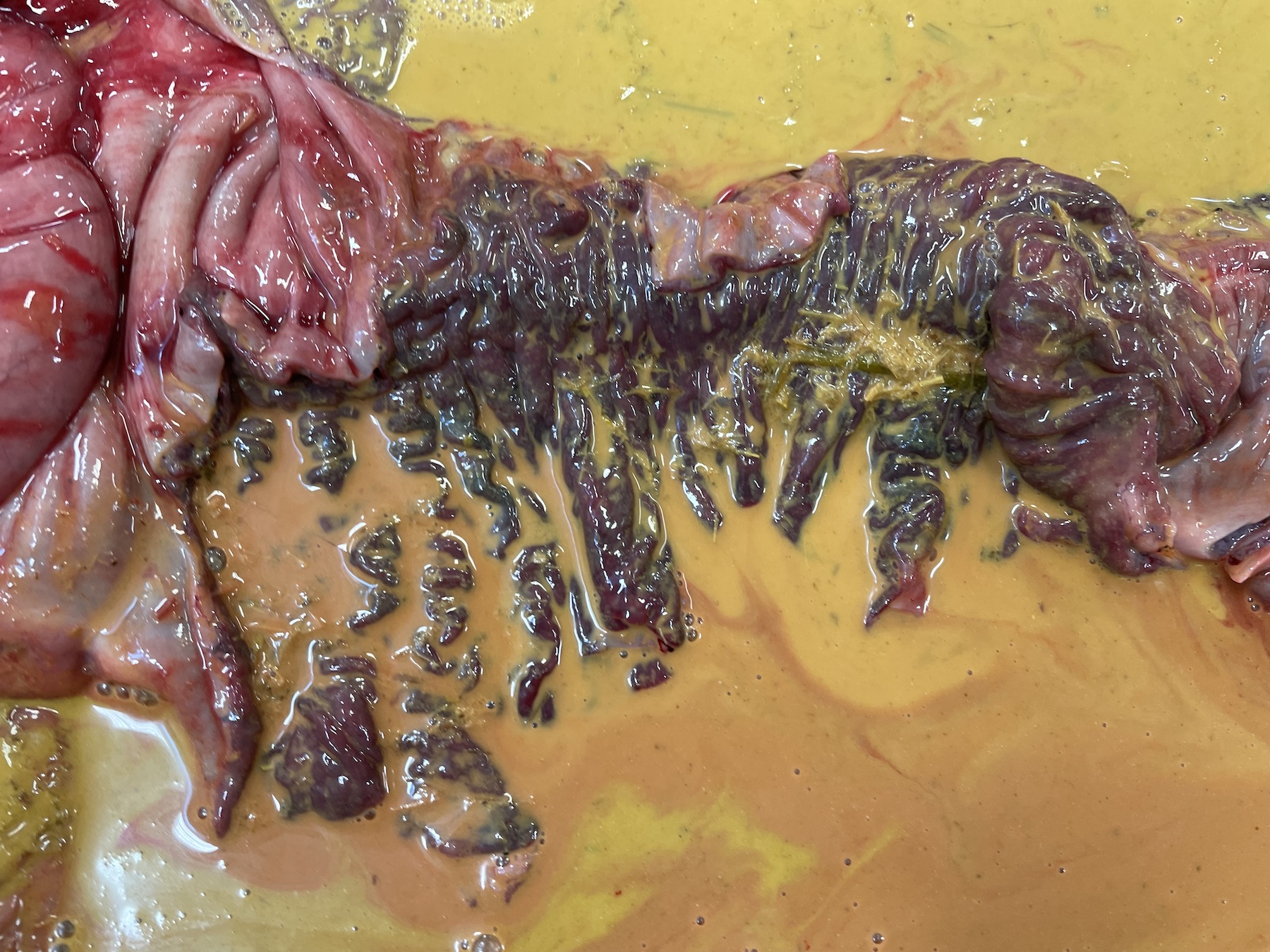
On postmortem examination, the foal was in good body condition. The sclera and mucous membranes were mildly yellow (icterus). The liver was diffusely enlarged, mottled pale red to dark purple with disseminated pinpoint to ~1 mm diameter pale tan foci scattered throughout all lobes. The wall of the right dorsal colon was moderately thickened by edema, and the mucosa was diffusely dark purple. The right dorsal colon contained a moderate amount of opaque, homogeneous, bright yellow, watery digesta. The small colon contained small, soft, partially formed fecal balls. The skeletal muscle was diffusely pale tan to red. The lungs were slightly wet, heavy and mottled pale pink to dark red. On section, they oozed a small amount of serous fluid. A complete postmortem was performed, and no other significant gross lesions were identified.
Laboratory Results:
|
CBC |
|
|
Neutrophils |
100 cells/µl (3,000-7,000) |
|
Bands |
400 cells/µl (0-100) |
|
Fibrinogen |
800 mg/dL (100-400) |
|
Chemistry |
|
|
AST |
1593 IU/L (185-375) |
|
T-bilirubin |
3.3 mg/dL (0.4-1.8) |
|
SDH |
223 IU/L (0-10) |
|
GGT |
54 IU/L (10-25) |
|
Glucose |
62 mmol/L (70-135) |
|
Sodium |
123 mEQ/L (132-142) |
|
Chloride |
84.4 mEQ/L (97-104) |
|
Bicarb |
20 mEQ/L (26-33) |
|
Anion gap |
22 mmol/L (8-15) |
|
Icterus |
6 mg/dL (1-3) |
|
Lactate |
7.2 mmol/L (1.11-1.78) |
|
Bile acids |
12 µmol/L (0-8.0) |
PCR testing performed on fresh liver tissue at the University of Kentucky for Clostridium piliforme was positive.
PCR testing on fresh feces performed at University of California Davis was negative for the following: Clostridium difficile toxin A and B, Lawsonia intracellularis, Salmonella spp., Cryptosporidium spp., Rhodococcus equi, Clostridium perfringens antigen, C. perfringens alpha toxin, C. perfringens beta toxin, C. perfringens beta2 toxin, C. perfringens cytotoxin netF, and C. perfringens enterotoxin. PCR testing for C. piliforme was not performed on the feces.
Microscopic Description:
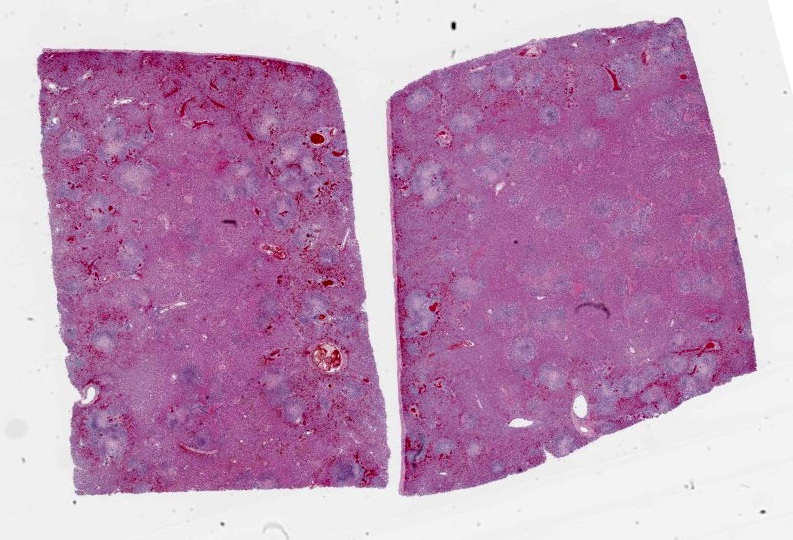
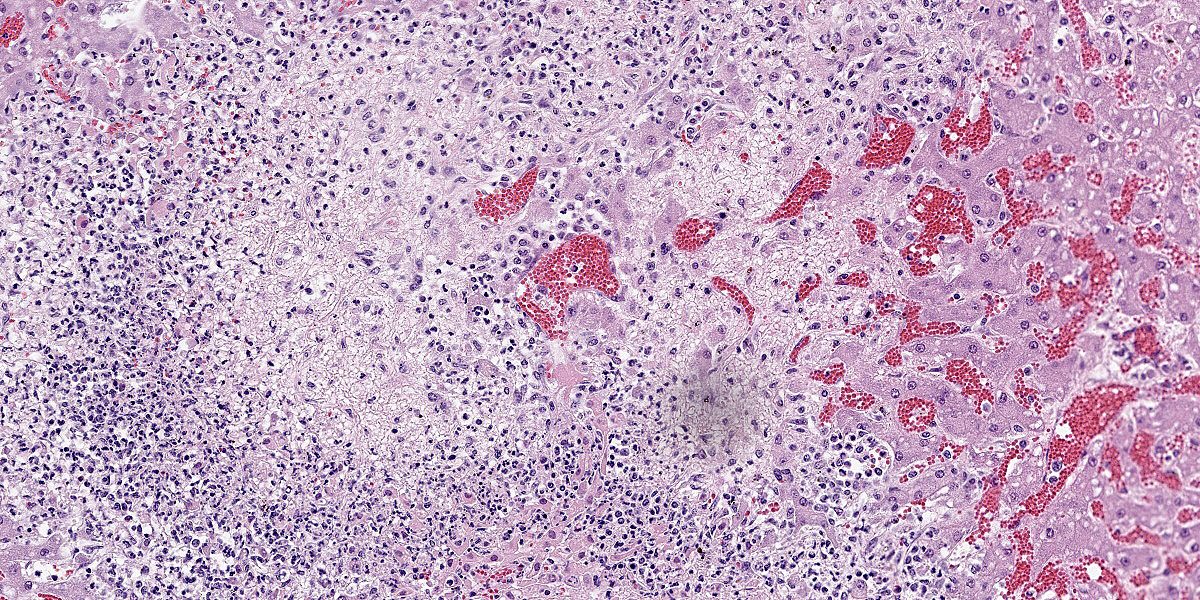
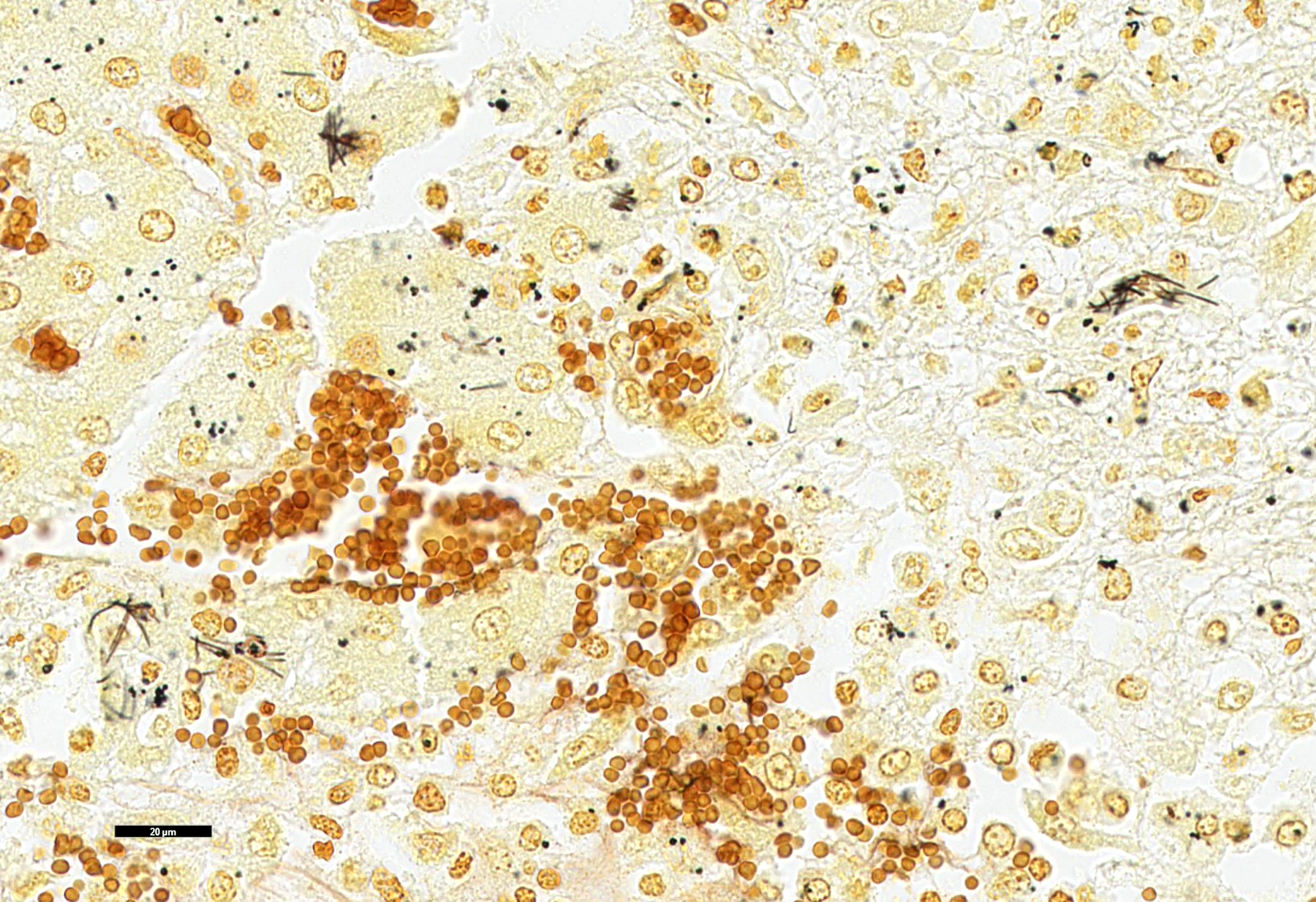
Liver: Multifocally and randomly affecting approximately 80% of the hepatic parenchyma are numerous 150-300 micron diameter foci of lytic necrosis characterized by fibrin, cellular debris, and high numbers of degenerate neutrophils. Necrotic foci are often coalescent, forming larger discrete sites (up to ~1 mm) of necrosis. There are hypereosinophilic and shrunken necrotic hepatocytes with pyknotic or karyorrhectic nuclei at the interface between necrotic and viable tissue. Peripheral to regions of necrosis, less affected hepatocytes occasionally contain numerous intracytoplasmic, stacked, pale, basophilic, filamentous bacilli. The portal interstitium is expanded by edema and variable numbers of lymphocytes and plasma cells. Few neutrophils and lymphocytes infiltrate through the hepatic capsule, which is also mildly edematous. The mesothelium is mildly reactive. Steiner’s stain reveals numerous, stacked, argyrophilic elongate bacilli within hepatocytes at the periphery of necrotic foci.
Contributor’s Morphologic Diagnosis:
Liver: Severe acute multifocal random necrosuppurative hepatitis with intrahepatocellular argyrophilic filamentous bacilli, etiology consistent with Clostridium piliforme.
Contributor’s Comment:
Tyzzer’s disease is a bacterial infection caused by Clostridium piliforme, an anaerobic, generally gram-negative, argyrophilic, pleomorphic, obligate intracellular bacillus. It can cause clinical disease in a variety of animals, including rodents, foals, calves, dogs, nonhuman primates, and cats.5 One case of Tyzzer’s disease has also been reported in an HIV-1 infected human.7 Clinical disease generally occurs in young or immunocompromised animals and can cause lethargy, fever, anorexia, icterus, seizures, coma and death.8 Foals generally succumb to fatal disease between 2 and 4 weeks of age.8
The pathogenesis of Tyzzer’s disease is not fully elucidated. However, it is postulated that fecal-oral transmission of spores leads to gastrointestinal infection of mucosal enterocytes in the ileum, cecum and colon. Following necrotizing enteritis/typhlocolitis, portal circulation of bacteria leads to hepatic and systemic involvement.6 Adult horses are generally resistant to disease but can harbor and shed C. piliforme in their feces. Foals commonly consume the dam’s feces within the first few weeks of life, and it is likely that in many cases contaminated feces from the dam is a primary source of infection.8 However, spores of C. piliforme can also persist in the environment for at least 5 years, so long-term environmental contamination can also be problematic.1
Tyzzer’s disease classically causes a triad of hepatitis, colitis and myocarditis. The prevalence of each of these lesions varies between species. In foals, the most common finding is multifocal necrotizing hepatitis that is occasionally accompanied by necrotizing lymphohistiocytic colitis and less frequently a multifocal necrotizing myocarditis. In a recent case series of 25 cases, all foals had hepatitis, 10/25 had colitis, and only 8/25 had myocarditis.3 The triad of concurrent lesions is more commonly observed in rodents and lagomorphs.6 In this case, there was necrotizing hepatitis, a mild to moderate necrotizing lymphohistiocytic colitis and mild patchy gray matter edema with proliferation of Alzheimer type II astrocytes. There were no remarkable microscopic findings in the myocardium. The changes in the brain are compatible with hepatic encephalopathy. Hepatic encephalopathy in cases of Tyzzer’s disease results from hyperammonemia secondary to acute liver failure. Horses, in contrast to carnivores and ruminants, do not exhibit spongy vacuolation of myelin (status spongiosis) with hyperammonemia.4
The diagnosis of Tyzzer’s disease can be made with gross and histologic findings with the presence of intracellular filamentous bacteria within hepatocytes. The bacteria can be faintly visualized on H&E and highlighted by silver stains (such as Steiner’s or Warthin-Starry), Giemsa, and periodic acid-Schiff stains.6 PCR amplification of the 16S rRNA gene of C. piliforme can be performed to further support Tyzzer’s disease. Considering the close phylogenetic proximity to other nonpathogenic clostridia, such as C. colinum, the PCR results should be interpreted with the gross and histologic findings. C. piliforme is very difficult to culture but can be isolated by inoculation of embryonated chicken eggs or specific primary cell lines.1
Other differentials that may be considered with multifocal necrosuppurative hepatitis without distinct intracellular filamentous bacteria in a foal include EHV-1 or bacteria that lead to septicemia (e.g. Salmonella spp., Actinobacillus equuli, Listeria monocytogenes). Other causes of foal diarrhea include equine rotavirus, equine coronavirus, Rhodococcus equi, Clostridium perfringens, Clostridium difficile, Lawsonia intracellularis, and Cryptosporidium spp.
Contributing Institution:
National Institutes of Health Comparative Biomedical Scientist Training Program (CBSTP) in collaboration with Colorado State University Veterinary Diagnostic Laboratory
https://nih-cbstp.nci.nih.gov/
https://vetmedbiosci.colostate.edu/vdl/
JPC Diagnosis:
Liver: Hepatitis, necrotizing, multifocal to coalescing, random, with occasional intracytoplasmic bacilli.
JPC Comment:
The contributor provides an excellent, thorough review of Tyzzer disease, a well-known and well-described clostridial disease of wide veterinary importance. Less well-known, sadly, is the fascinating biography of Dr. Ernest Edward Tyzzer, the scientific Renaissance man for whom Tyzzer disease is named.
Dr. Tyzzer was born in a suburb of Boston in 1875 and paid his way through college and Harvard Medical School by trapping muskrat, fox, mink, skunk, and weasels.10 His pathology and parasitology-steeped career began in his second year of medical school when, during one of his trapping trips, he discovered parasites in a fox carcass and brought the carcass to class, endearing himself to the pathology faculty (though perhaps less so to his fellow students).
His curiosity about the natural world led to a broad study of infectious agents in a variety of species. Immediately after graduating from medical school, Tyzzer began studying the histologic lesions of varicella virus and determined that the intranuclear inclusions generated in the disease were likely viral in nature and not morphologic stages of an unknown protozoal parasite, the accepted theory at the time.10 Tyzzer spent the next eleven years as the Director of Research for the Harvard Cancer Commission, where he investigated the occurrence of spontaneous murine tumors, host responses to transplantable tumors, and the biology of metastasis. During this time, three mice supplied by Tyzzer to one of his research collaborators became the founding members of the DBA inbred strain of mice now commonly used in medical research.10
Dr. Tyzzer also discovered and described the first Cryptosporidium species, C. muris, in the gastric glands of laboratory mice in 1907. Two follow-on publications in 1910 and 1912 contained further descriptions, gleaned solely from light microscope observations, about the life cycle and biology of C. parvum and C. muris that are still largely accepted today.9
Tyzzer was also responsible for correctly classifying the causative agent of blackhead in turkeys, naming it Histomonas meleagridis, and identifying that ingestion of contaminated nematode eggs was the connection between Histomonas meleagridis and Heterakis spp.10 Having worked out the pathogenesis of the disease, Tyzzer then set up an experimental turkey farm where he worked out effective management practices to produce healthy turkeys and outlined these practices in pamphlets distributed to farmers by the Massachusetts Department of Agriculture. Tyzzer received a citation from the Governor of Massachusetts for this work, crediting him for saving the Massachusetts turkey industry.10
Dr. Tyzzer was involved in the discovery and elucidation of many other conditions not described above, including Tyzzer’s disease, the causative agent of which he first described in a colony of Japanese waltzing mice in 1917. Tyzzer was also known for his humor, which was on display after he published a case report on his very own bleeding rectal polyp and accompanying Entamoeba coli infection. Though never identifying the patient, his manuscript stated that no systematic follow up study could be arranged “owing to the non-cooperative attitude of the patient”.10
This week’s slide conference, moderated by MAJ Kelsey Fiddes, Chief of Resident Training at the Joint Pathology Center, kicked off with this classic presentation of Dr. Tyzzer’s eponymous disease. As is typical of this disease, the characteristic filamentous bacilli are covertly stacked within the cytoplasm of hepatocytes near the outside edge of necrotic lesions and require some imagination to appreciate with H&E staining alone. Silver stains such as Steiner’s and Warthin-Starry allowed visualization of these bacteria and their classic “haystack” appearance.
Conference discussion focused mainly on differences in clinical presentations among various species. The classic “heart, intestine, liver” triad of lesions repeated breathlessly by countless pathology residents is seen mainly in laboratory animals, while horses manifest their Tyzzer mainly through necrotizing hepatitis, as in our case. Clostridium piliforme infection in rats is associated with megaloileitis and with encephalitis in gerbils. Finally, a 2023 retrospective study found that the most common manifestation of Tyzzer disease in kittens is ulcerative colitis, followed by hepatitis and perianal dermatitis.2
References:
- Barthold SW, Griffey SM, Percy DH. Mouse. Pathology of laboratory rodents and rabbits. 4th ed. Wiley;2016:1–118.
- Fingerhood S, Mendonca FS, Uzal FA. Tyzzer disease in 19 preweaned orphaned kittens. J Vet Diagn Invest. 2023;35(2): 212-216.
- García JA, Navarro MA, Fresneda K, Uzal FA. Clostridium piliforme infection (Tyzzer disease) in horses: retrospective study of 25 cases and literature review. J Vet Diagn Invest. 2022;34(3):421–428.
- Hasel KM, Summers BA, De Lahunta A. Encephalopathy with idiopathic hyperammonaemia and Alzheimer type II astrocytes in Equidae. Equine Vet J. 1999; 31(6):478–482.
- Jubb KVF, Kennedy PC, Palmer N. Pathology of domestic animals. Academic press; 2012.
- Navarro MA, Uzal FA. Pathobiology and diagnosis of clostridial hepatitis in animals. J Vet Diagn Invest. 2020;32(2): 192–202.
- Smith KJ, Skelton HG, Hilyard EJ, et al. Bacillus piliformis infection (Tyzzer’s disease) in a patient infected with HIV-1:
- confirmation with 16S ribosomal RNA sequence analysis. J Am Acad Dermatol. 1996;34(2):343–348.
- Swerczek TW. Tyzzer’s disease in foals: retrospective studies from 1969 to 2010. Can Vet J. 2013;54(9):876.
- Tzipori S, Widmer G. A hundred-year retrospective on cryptosporidiosis. Trends Parasitol. 2008;24(4):184-189.
- Weller TH. Ernest Edward Tyzzer 1875-1965. National Academy of Sciences; 1978.