Signalment:
Gross Description:
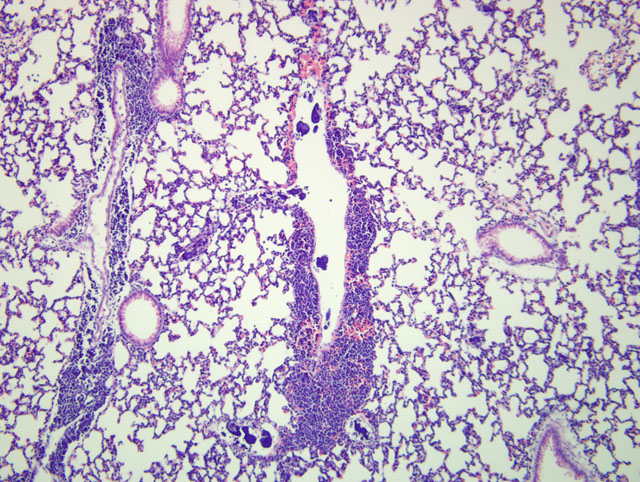
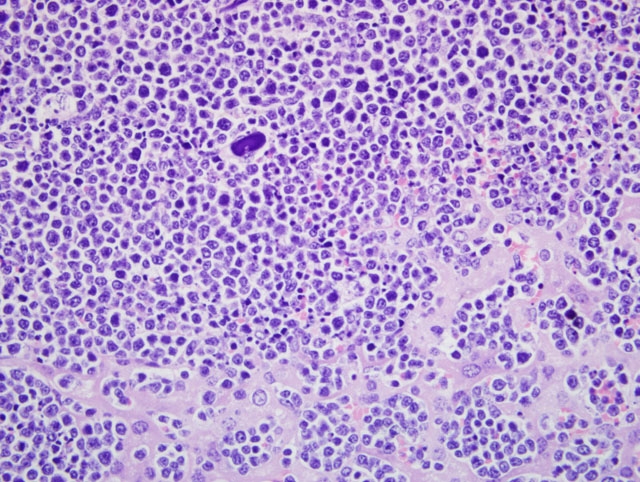
Histopathologic Description:
Liver: There is severe infiltration of neoplastic cells as described above. Diffusely, the cells fill and markedly expand the sinusoids and compress the hepatic plates. They form confluent aggregates, especially around large blood vessels. Cellular debris is common. Vascular lumina contain a large number of neoplastic cells and occasional basophilic emboli as described above.
Morphologic Diagnosis:
Condition:
Contributor Comment:
In humans, ATLS is caused by rapid lysis of malignant cells leading to a massive release of cellular contents. The large amount of cellular debris overwhelms homeostatic mechanism and causes an acute metabolic crisis.(1,2,3) Spontaneous ATLS, as in this case, is rare.(1) Most cases are reported in association with aggressive chemotherapy or radiotherapy.(1,3) Acute tumor lysis syndrome is more common with hematologic malignancies than with solid tumors.(1,3) Clinically, the disorder is characterized by severe metabolic abnormalities, including hyperuricemia, hyperphosphatemia and hyperkalemia, due to substantial breakdown of nucleic acids and their release from dead tumor cells along with phosphate and potassium.(1,2,3) These abnormalities may lead to acute renal failure (the most commonly reported cause of death in human ATLS patients), bradyarrhythmias and cardiac arrest.(1,3)
In the veterinary literature, there are a several case reports of chemotherapy-associated ATLS in dogs, a cat, and in a 129/SvEv mouse with PML/RARα-induced acute myeloid leukemia.(3) Spontaneous ATLS was reported in a DBA/1J mouse with leukemic lymphoma.(1) The current case is another example of spontaneous ATLS. As postulated by others, it is possible that transfer of this mouse out of the animal house led to a stress-induced surge of endogenous glucocorticoids which precipitated fatal ATLS.(1)
In common with the two other murine cases, the emboli in this case were most prominent in the pulmonary vasculature.(1,3) In the case from 2003, other than the lung, emboli were identified in virtually all tissues, but were especially common in the kidney and brain.(1) In the case from 2009, emboli were observed in multiple organs, but other than the lung, were especially prominent in the liver, as in the submitted case.(3) In the submitted case, a low number of emboli in the kidney and rare emboli in lymph nodes were also observed.
In the current case, quantification of cellular death was not undertaken. Cellular debris was common, but in our slides, the number of viable cells far exceeded that of dead cells. In the other murine report of spontaneous ATLS, necrosis affected up to 90% of tumor cells.(1) In the report from 2009, caspase-3 labeled cells are described as plentiful.(3)
Electron microscopy was done on one of the murine cases.(1) The basophilic emboli had uniform electron dense appearance similar to that of nuclear chromatin in apoptotic lymphocytes. In mixed emboli, which at the light microscopic level had a variegated basophilic and eosinophilic appearance, the eosinophilic component was composed of aggregated cytoplasmic fragments of necrotic tumor cells.(1)
Interestingly, the authors of the 2003 report in a mouse claim to be the first to describe widely disseminated DNA and cellular debris in ATLS in humans or any other species. They ascribe this to the fact that most reports of ATLS in humans are focused on clinical management and that the few reports which describe the pathologic features of this condition are based on lesions found at death, which typically occurs several days to weeks after the onset of ATLS.(1) At that point, most reported lesions are renal and attributed to urate crystal deposition in the medulla.(1)
As noted above, in the 2003 report, emboli were identified in virtually all tissues. The authors propose that mechanical obstruction of capillary beds by these emboli plays an important role in the pathogenesis of ATLS.(1) In the more recent report of ATLS, the cause of death was postulated to be respiratory failure following massive embolization of chromatin clumps and necrotic debris in pulmonary vasculature.(3)
JPC Diagnosis:
Conference Comment:
The underlying cause of lysis of neoplastic cells is, as of yet, undetermined. One hypothesis is that the release of an endogenous substance may cause lysis of neoplastic cells; a suspected agent is corticosteroids, which are commonly administered for the treatment of lymphoma. Once lysed, neoplastic cells release nucleic acids, potassium, and phosphorus, resulting in the observed clinicopathologic abnormalities outlined by the contributor. The nucleic acids are broken down into their respective purine and pyrimidine components. Purines are further metabolized to uric acid, which is then converted to allantoin via urate oxidase and subsequently excreted in the urine. Interestingly, humans and non-human primates lack urate oxidase and may develop hyperuricemia and uric acid crystals within renal tubules.(4,5)
The hallmark histologic finding of ATLS is that of widely dissemination microemboli of nuclear and cytoplasmic debris.(5) Microemboli are most commonly observed in the lung, kidneys, and brain. In one study, lesions of ATLS were more common in animals found dead as opposed to those that appeared sick at the time of necropsy.(4)
References:
2. Mylonakis ME, Koutinas AF, Papaioannou N, Lekkas S. Acute tumour lysis syndrome in a dog with B-Cell multicentric lymphoma. Aust Vet J. 2007;85:206-208.
3. Radaelli E, Marchesi F, Patton V, Scanziani E. Diagnostic exercise: sudden death in a mouse with experimentally induced acute myeloid leukemia. Vet Pathol. 2009:46:1301-1305.
4. Treuting PM, Albertson TM, Preston BD. Case series: acute tumor lysis syndrome in mutator mice with disseminated lymphoblastic lymphoma. Toxicol Pathol. 2010;38:476-485.
5. Vogel P, Pletcher JM, Liang Y. Spontaneous acute tumor lysis syndrome as a cause of early deaths in short-term carcinogenicity studies using p53+/- mice. Vet Pathol. 2010;47:719-724.