Signalment:
Gross Description:
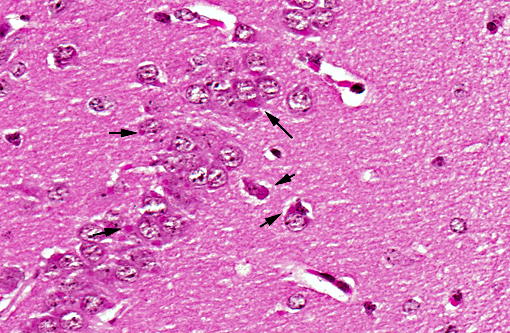
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
The genome of RV comprises five genes encoding the viral proteins nucleoprotein, phosphoprotein, matrix protein, glycoprotein and the viral RNA polymerase. The replication of RV proceeds in the presence of the host immune response, including the antiviral type I interferon (IFN) system. RV is known for its IFN-sensitivity and therefore must encode mechanisms that prevent IFN expression.5 It is hypothesized that the viral phosphoprotein is involved in this mechanism.(1)
Rabies occurs in more than 150 countries and territories worldwide. Dogs are the source of infection in all of the estimated 55, 000 human rabies deaths annually in Asia and Africa. Bats are the source of most human rabies deaths in the United States of America and Canada. Bat rabies has also recently emerged as a public health threat in Australia, Latin America and Western Europe. Infections due to contact to rabid foxes, raccoons, skunks, jackals, mongooses and other wild carnivore are very rare. Human-to-human transmission by bite has never been confirmed.(23)
RV is the causative agent of the classical rabies and is responsible for the vast majority of all human rabies cases. The transmission occurs via the bite of rabid animals shedding virus within their saliva, or direct contact of infectious material (i.e. saliva, cerebrospinal liquid, nerve tissue) to mucous membranes or skin lesions. The virus cannot penetrate intact skin. Rabies virus replicates in striated muscle cells or infects nerve cells, followed by a retrograde transport of virions to the central nervous system (CNS). Within the CNS, virus replication induces pathologic effects on nerve cell physiology. The anterograde transport of virus to secretory tissues of salivary glands leads to viral shedding via saliva.(24)
In animals, RV infection brings out neurological signs which might differ slightly depending on the species and the time point infected. The incubation period for rabies is typically 13 months, but may vary from <1 week to >1 year.(23) After this so-called prodromal stage, the onset of clinical symptoms follows, which lasts for about 1-3 days with aggressiveness, daytime activities in nocturnal animals or abnormalities in appetite. Potentially, the prodromal stage is followed by the excitative phase with severe agitation and aggressiveness. Further, in the paralytic phase, the infected animals are unable to swallow, leading to a typical sign of foaming saliva around the mouth. A complete paralysis occurs and is followed by death.(24)
Many neurotropic viruses use apoptosis as a mechanism of neuropathogenicity.(16) The induction of apoptosis by rabies virus has been a controversial topic, but increasing evidence indicates that pathogenic rabies virus strains do not induce apoptosis.(8)
In natural rabies, pyknotic chromatolytic neurons are seen throughout the CNS most frequently in the brainstem, and periaqueductal gray matter, cervical spinal cord, thalamus and less frequently the cerebral cortex.(4) In general, these degenerated neurons do not harbor Negri bodies but are positive for viral antigen and may be accompanied by inflammatory reaction.(6) As in other viral encephalitides, neuronophagia and glial nodules are seen often in areas where neuronal degeneration and inflammatory cell infiltration are conspicuous. Topographic dissociation between location of inflammatory reactions and of Negri body has been reported in an autopsy study of 49 cases. Inflammatory changes were most frequently found in medulla, pons and spinal cord followed by thalamus and less frequently in cerebellum and hippocampus. Negri body bearing neurons were rare where inflammation was dense.(10) Perivascular infiltrates are composed of lymphocytes and monocytes intermingled with small numbers of granulocytes and plasma cells, depending on the stage and severity of infection.(7)
Two different mechanisms have been proposed for the transport of rabies virus through the axon to the cell body: transport of either the rabies virus capsid alone or transport of the whole virion. A recent study showed that the whole virion is transported to the cell body in an endosomal vesicle, although the exact mechanism by which this occurs remains unclear.(12) The rabies virus glycoprotein mediates the entry of the virion into the vesicle. However, as the rabies virus glycoprotein is inside the vesicle, it should not be able to interact directly with a specific transporter complex. Based on these observations, it is speculated that entry into the vesicle determines the direction and provides the driving force of rabies virus transport. This indicates that the nature of the vesicle formed dictates the transport method that follows.(19)
In cases of human rabies, the patients die with absence of protective antibodies. The immune privileged status of the CNS and the bloodbrain barrier might explain the delayed development of a protective immune response and the poor survival rate.(10) Remarkably, there is one report of a human survivor of rabies. The patient had detectable antibody and the treatment applied was aimed to allow the humoral immune response to develop. High IgG titres developed and might have contributed to the survival of this patient.(24)
The influence of the immune privileged status of the CNS on the antibody response is controversial. In contrast to RV, Herpes viruses and Borna disease virus can infect the brain, but are effectively controlled by the immune system. Several other factors are discussed, as for example that the infectious dose transmitted with a bite is too small to trigger immune responses and further enable the virus to infect sensory nerves. A final explanation for the lack of antibody could result from immunosuppression induced by the virus. It is known that the viral phosphoprotein inhibits interferon response. However, RV infection is associated with the increase in gene transcripts for interferon-inducible genes. It is speculated that interferon inhibition is transitory and provides a short delay in the host response.(10)
Table: Current classification of the Lyssavirus genus, host species and geographical distribution.(11)
| Virus and Genotype (I-VII) | Hosta | Geographical distribution |
| Classical rabies virus (RABV), I | Numerous chiropteran and carnivoran species | Worldwide |
| Lagos bat virus (LBV), II | Epomophorus wahlbergi (Wahleberg's epauletted fruit bat) | Africa |
| Mokola virus (MOKV), III | Unknown | Africa |
| Duvenhage virus (DUVV), IV | Miniopterus species (?) | Southern Africa |
| European bat lyssavirus type 1 (EBLV- 1), V | Eptesicus serotinus (Serotine bat) | Europe |
| European bat lyssavirus type 2 (EBLV-2), VI | Myotis daubentonii (Daubenton's bat) | Western Europe |
| Australian bat lyssavirus (ABLV), VII | Australian mega- and microchiropteran species | Australia |
| Aravan | Myotis blythi (lesser mouse-eared bat)b | Kyrgistan |
| Khujand | Myotis mystacinus (whiskered bat)b | Kyrgistan |
| Irkut | Murina leucogaster (greater tube-nosed bat)b | Eastern Siberia |
| West Caucasian bat virus (WCBV) | Miniopterus schreibersi (common bent-winged bat)b | Western Caucasus Mountains |
b Single isolations have been made.
JPC Diagnosis:
Conference Comment:
Australian bat lyssavirus, mentioned by the contributor, is a virus closely related to rabies that was discovered in Australia in 1996. It occurs in various species of flying foxes and occasionally in other species of bats and is responsible for a few human fatalities. Lesions are similar to rabies and consist of nonsuppurative meningoencephalomyelitis and ganglioneuritis with Negri bodies(6).
Pseudo-Negri bodies, which may be confused with rabies and are a reason that rabies diagnosis should not be based solely on the presence of Negri bodies, are found in cats, skunks, and dogs and are nonspecific, 1.5 um, homogenous inclusions in the pyramidal cells of the hippocampus. They present in cats as nonspecific inclusions in the lateral geniculate neurons; in dogs as cytoplasmic lamellar bodies in the thalamic neurons and Purkinje cells; in aged sheep and cattle as nonspecific 1 um, brightly eosinophilic, angulated inclusions in the large neurons of the medulla and spinal cord; in Japanese brown beef cattle; in mice as hippocampal inclusions; and in woodchucks as inclusions in the brainstem(3, 15, 17, 18, 20, 22).
References:
2. Cheville NF. Cytopathology of viral diseases. In: Ultrastructural Pathology, the Comparative Cellular Basis of Disease. 2nd ed. Ames, IA: Wiley-Blackwell; 2009:367-8.
3. Coetzer JAW, Tustin RCSwanepoel R: Rabies. In: Infectious Diseases of Livestock, ed. Coetzer JAW, Tustin RC, 2nd ed., vol. 2., pp.11231182. Oxford University Press, Cape Town, Africa, 2004.
4. Dupont JR, Earle KM. Human Rabies Encephalitis - a Study of 49 Fatal Cases with a Review of Literature. Neurology. 1965;15(11):1023-7.
5. Finke S, Conzelmann KK. Replication strategies of rabies virus. Virus Res. Aug 2005;111(2):120-131.
6. Fraser GC, et al. Encephalitis caused by a Lyssavirus in fruit bats in Australia. Emerg Infect Dis. 1996; 2(4):327-31.
7. Iwasaki Y, Liu DS, Yamamoto T, Konno H. On the Replication and Spread of Rabies Virus in the Human Central Nervous-System. J Neuropath Exp Neur. 1985;44(2):185-195.
8. Iwasaki Y, Sako K, Tsunoda I, Ohara Y. Phenotypes of Mononuclear Cell Infiltrates in Human Central-Nervous-System. Acta Neuropathol. May 1993;85(6):653-657.
9. Jackson AC, Rossiter JP. Apoptosis plays an important role in experimental rabies virus infection. J Virol. Jul 1997;71(7):5603-5607.
10. Johnson N, Cunningham AF, Fooks AR. The immune response to rabies virus infection and vaccination. Vaccine. May 21 2010;28(23):3896-3901.
11. Johnson N, Vos A, Freuling C, Tordo N, Fooks AR, Muller T. Human rabies due to lyssavirus infection of bat origin. Vet Microbiol. May 19 2010;142(3-4):151-159.
12. Klingen Y, Conzelmann KK, Finke S. Double-labeled rabies virus: Live tracking of enveloped virus transport. J Virol. Jan 2008;82(1):237-245.
13. Marston DA, McElhinney LM, Johnson N, et al. Comparative analysis of the full genome sequence of European bat lyssavirus type 1 and type 2 with other lyssaviruses and evidence for a conserved transcription termination and polyadenylation motif in the G-L 3 ' non-translated region. J Gen Virol, Apr 2007;88:1302-1314.
14. Maxie MG, Youssef S. Nervous system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 1., pp. 283457. Elsevier, Philadelphia, PA, 2007.
15. Mori I, Nishiyama Y, Yokochi T, Kimura Y. Virus-induced neuronal apoptosis as pathological and protective responses of the host. Rev Med Virol. Jul-Aug 2004;14(4):209-216.
16. Nietfeld JC, Rakich PM, Tyler DE, Bauer RW: Rabies-like inclusions in dogs. J Vet Diagn Invest 4:333338, 1989.
17. Pierce V, Snyder RL and McGrath JT: Intracytoplasmic neuronal inclusions in Woodchuck brain stem. J Wild Dis 26(1): 62-66, 1990
18. Schnell MJ, McGettigan JP, Wirblich C, Papaneri A. The cell biology of rabies virus: using stealth to reach the brain. Nat Rev Microbiol. Jan 2010;8(1):51-61.
19. Summers B, Cummings JF, De Lanhunta A: Inflammatory diseases of the central nervous system. In: Veterinary Neuropathology, ed. Summers B, Cummings JF, De Lanhunta A. 95188. Mosby, St. Louis, MO, 1995.
20. Tordo N, Poch O, Ermine A, Keith G, Rougeon F. Completion of the Rabies Virus Genome Sequence Determination - Highly Conserved Domains among the L (Polymerase) Proteins of Unsegmented Negative-Strand Rna Viruses. Virology. Aug 1988;165(2):565-576.
21. Whitfield SG, et al. A comparative study of the fluorescent antibody test for rabies diagnosis in fresh and formalin-fixed brain tissue specimens. J Virol Methods 95:145151, 2001.
22. WHO Rabies Feact Sheet No. 99. http://www.who.int/mediacenter/factsheets/fs099/en/. September 2010.
23. WHO Rabies Bulletin. http://www.who-rabies-bulletin.org/About_Rabies/Transmission_Pathogenesis.aspx.
24. Willoughby RE, Tieves KS, Hoffman GM, et al. Brief report - Survival after treatment of rabies with induction of coma. New Engl J Med. Jun 16 2005;352(24):2508-2514.