Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 12
December 13th, 2017
CASE III: C973 (JPC 4020995).
Signalment: 9 year-old male neutered greyhound, Canis familiaris, canine.
History: The animal presented with a 2-month history of progressive generalized ataxia and hypermetria in all 4 limbs. Multifocal left forebrain, cerebellar and brainstem signs were observed. Magnetic resonance imaging revealed multifocal white and grey matter lesions, notably worse on the left parietal lobe. Cerebrospinal fluid analysis revealed no significant abnormalities. Serology was positive for Toxoplasma gondii at 1:200 and Neospora caninum at 1:800. Treatment with steroids and clindamycin was initiated; however the dog deteriorated and was subsequently euthanized. Brain tissue was polymerase chain reaction (PCR) positive for Neospora caninum.
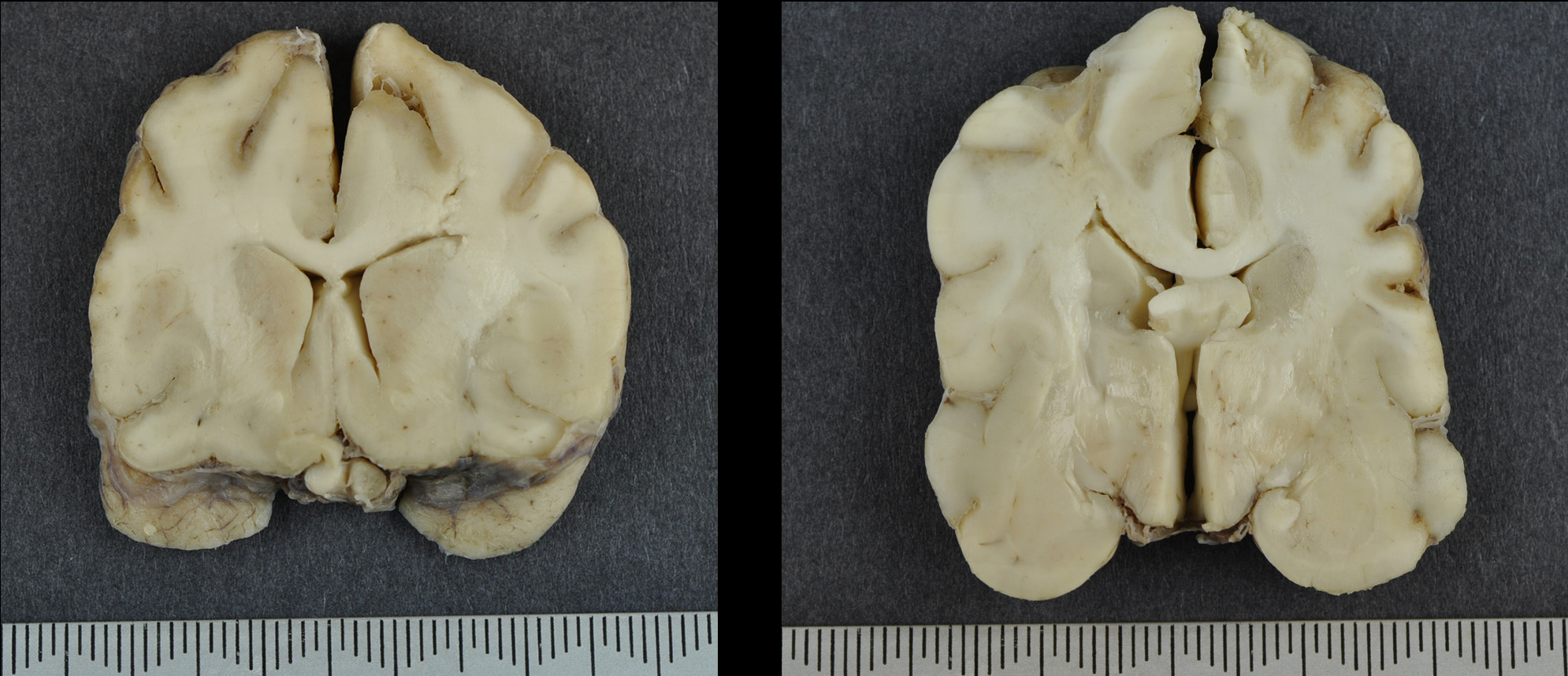
Gross Pathology: The brain was submitted for post mortem examination. The cerebral hemispheres appeared slightly asymmetrical. Prosection revealed expansion of the left frontal dorsal white matter. In multiple sections, the dorsal and lateral cerebral cortices were focally thinned and discoloured with a tan to brown appearance.
Laboratory results:
Serology was positive for Toxoplasma gondii at 1:200 and Neospora caninum at 1:800. Treatment with steroids and clindamycin was initiated; however the dog deteriorated and was subsequently euthanized. Brain tissue was polymerase chain reaction (PCR) positive for Neospora caninum.
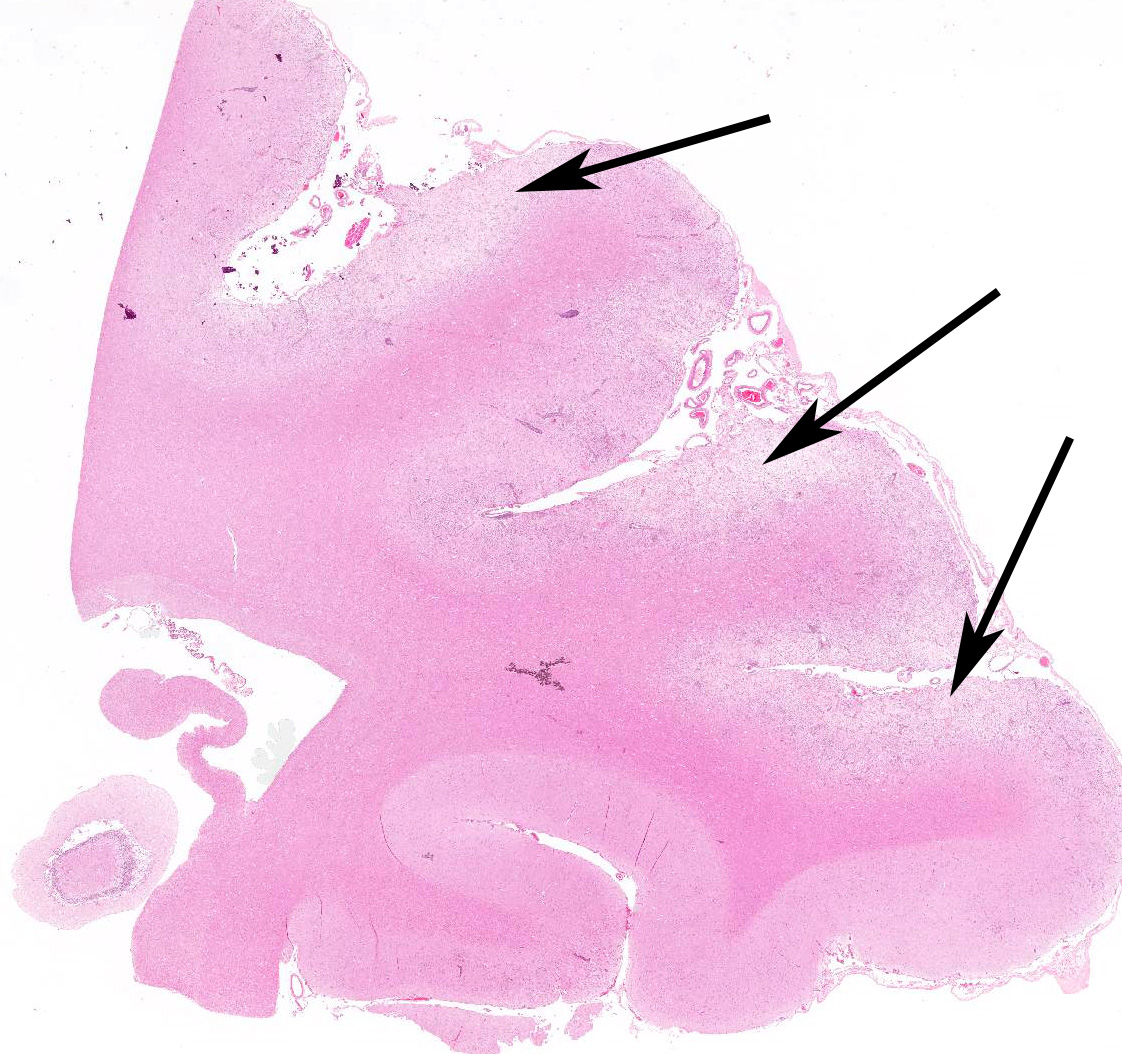
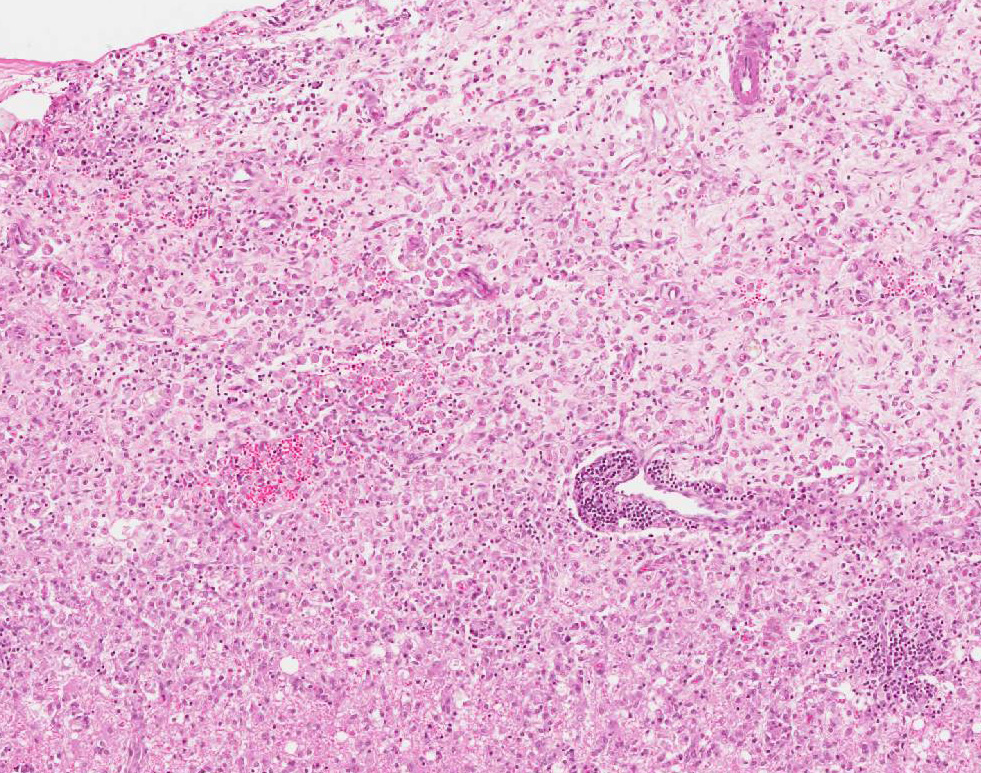
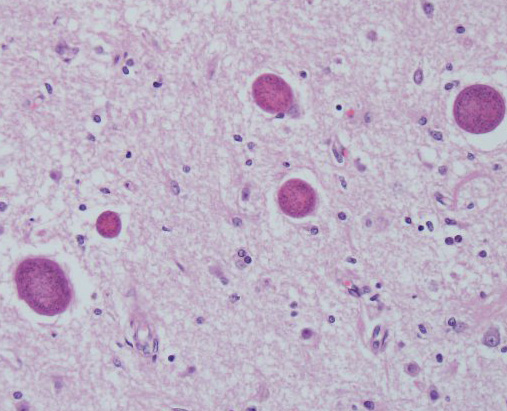
Microscopic Description: Multiple brain sections revealed a widespread, bilateral necrotizing inflammatory lesion affecting mainly the dorsal and dorsolateral cerebral cortices, characterised by multifocal extensive cerebrocortical necrosis. The necrotic grey matter was replaced by fibrillary gliosis or cavitated and contained many gitter cells, with some slides exhibiting more destructive lesions than others. Multifocal moderate to marked lymphocytic and lesser plasmacytic aggregates were seen within the leptomeninges and the necrotic cortices, forming variably sized perivascular cuffs. Multiple reactive (proliferating) vessels were observed. The corona radiata in areas appeared degenerate or necrotic with many areas of myelin vacuolation.
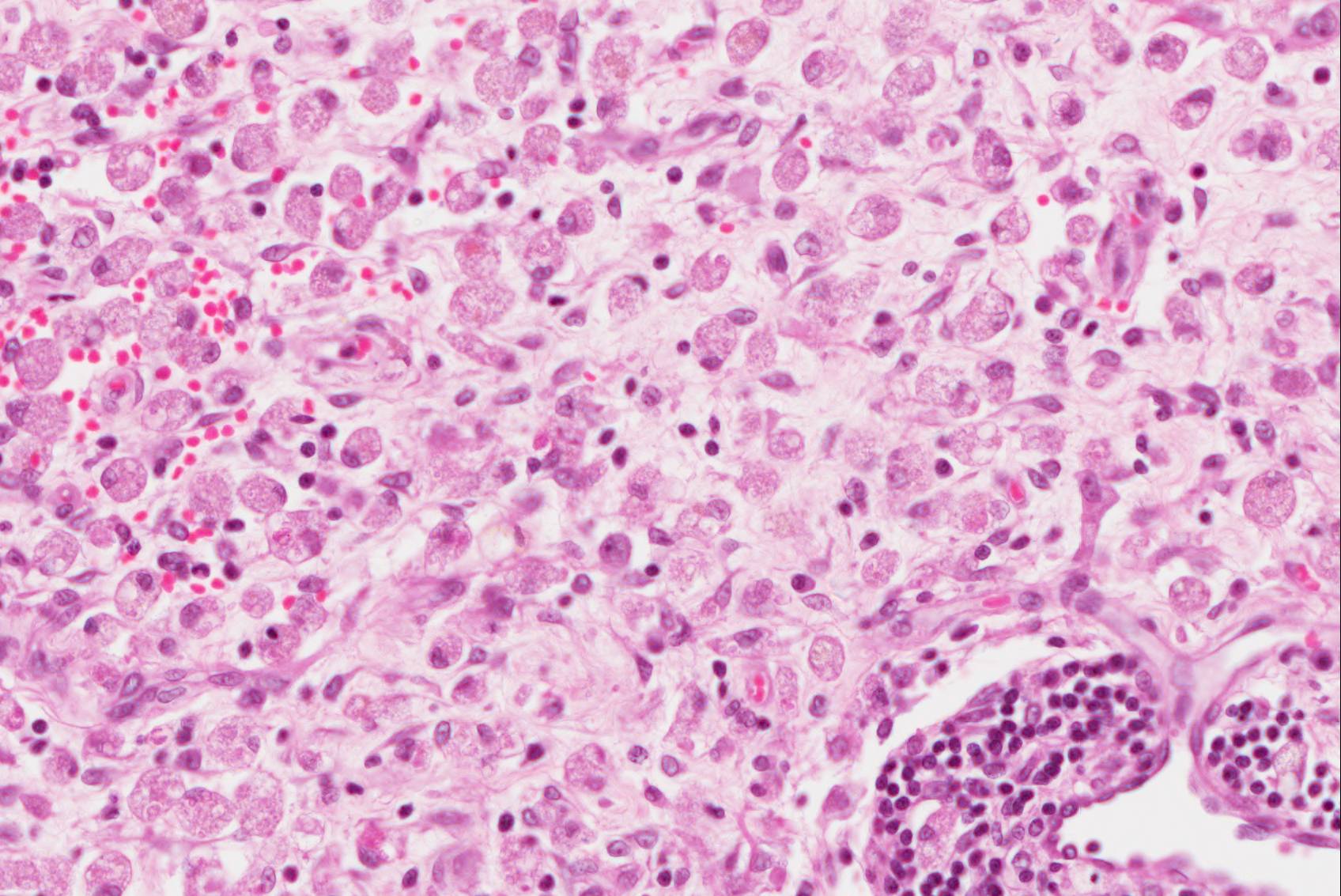
Found within and adjacent to the areas of cerebrocortical necrosis and more widely in the brain were abundant round to oval protozoal cysts measuring up to 90 um in diameter with a 2-4 um thick eosinophilic cyst wall enclosing numerous 2-3 um basophilic bradyzoites.
Additional sections
Minimal to milder inflammation and gliosis was observed surrounding the lateral, third and fourth ventricles and deeper tissue and within the mesencephalon. Severe bilateral degeneration characterised by myelin vacuolation, few intramyelinic macrophages, and gliosis was noted in the crus cerebri and pyramids. A small area of the cerebellar vermis exhibited vacuolation and some disruption of the cortex with mild loss of Purkinje neurons.
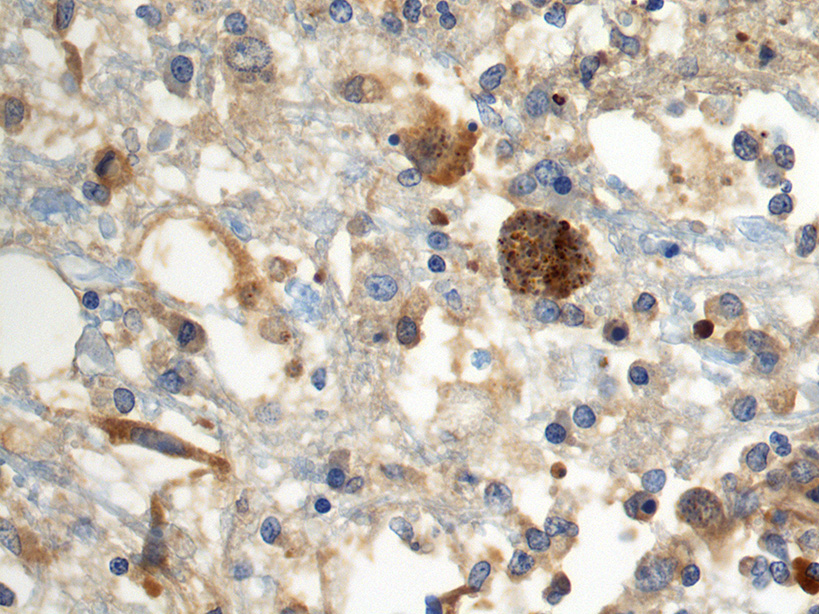
Immunohistochemistry: cysts within sections of the cerebrum were positive in stains for N. caninum.
Contributor’s Morphologic Diagnosis:
Brain, cerebral cortex: Meningoencephalitis, necrotizing, chronic-active, multifocally extensive, severe, with protozoal cysts.
Contributor’s Comment: Neospora caninum is a cyst-forming coccidian parasite in the phylum Apicomplexa, family Sarcocystiidae and like other coccidians, is an obligate intracellular parasite12,13.
Molecular analysis shows that N. caninum is closely related to Toxoplasma gondii12. Both coccidian parasites have proliferative (tachyzoite) and tissue cyst (bradyzoite) phases with tachyzoites proliferating by endodyogeny. Some differences in morphology and life cycle exist; N. caninum has a thicker cyst wall and does not develop within a host cell parasitophorous vacuole as does Toxoplasma gondii. Differentiation by light microscopy alone is unreliable. Electron microscopy, immunohistochemistry and molecular techniques are required for definitive diagnosis15. Co-infection with T. gondii can theoretically occur and it should be considered as a differential diagnosis8.
Naturally occurring neosporosis has been reported in a variety of animals including dogs, cats, cattle, sheep, goats, deer, water buffalo, antelope, a rhinoceros and horses (Neospora hughesi). In general, neosporosis is primarily a disease of cattle and dogs where the parasite causes abortion and CNS/PNS/muscle disease respectively5,6,7,15. The domestic dog is the definitive host for the parasite; however, experimental studies have shown that the Australian dingo and the coyote are also definitive hosts8,15. The mechanism of natural infection in dogs is incompletely understood, however ingestion of neural and muscle tissue containing cysts is considered the most likely source of infection8. In cattle, N. caninum is very efficiently transmitted transplacentally (vertically) but consumption of bovine foetal membranes can also act as a source of infection in dogs7. Transplacental transmission in the terminal stages of gestation and post-natal transmission via milk occurs in dogs. Infected dogs produce environmentally resistant oocysts which play an important role in the epidemiology of neosporosis14. To date, viable fecal oocysts have been demonstrated only in naturally infected dogs and the gray wolf8. The ingestion of sporulated N.caninum oocysts from the environment is the natural method of infection in juvenile and adult cattle which are the main intermediate hosts14.
The Neospora lifecycle involves three infectious stages: (1) Oocysts produced in the feces of dogs following ingestion of bradyzoites; (2) The tachyzoite, a rapid multiplying stage which initiates lesion development by multiplying in and rupturing cells; (3) with onset of host immune defense, tachyzoites differentiate into bradyzoites to form tissue cysts mainly in the central nervous system (CNS) and muscle of dogs and in the intermediate host2.
N. caninum tachyzoites can invade a variety of cell types in many organs including those of the monocyte-macrophage system. The most likely method of spread to the CNS occurs through infected leukocytes crossing the blood-brain barrier. Findings typical of pathogens with endothelial tropism such as vascular swelling and injury, tissue ischemia and multifocal infarction are often observed in CNS lesions caused by N. caninum14,15.
Clinical disease can affect dogs of all ages, however the most severe cases of N. caninum typically occur in congenitally infected puppies over 3 weeks of age8, involve several animals in a litter and present as a progressive ascending neuromuscular paralysis caused by encephalomyelitis, polymyositis and especially polyradiculitis1. Neurological signs are dependent on the site that is parasitized and may include such features as; rigidity following muscle denervation and contracture leading to rigid hyperextension of the pelvic limbs, cervical weakness, and dysphagia1,5. Pelvic limb contracture is one of the most consistently reported signs in pups8. Widespread involvement of the CNS and other organs occurs in adult dogs with signs of disseminated disease including pneumonia, polymyositis, myocarditis, dermatitis, and hepatitis1,5,15.
Gross CNS lesions can be present throughout the white and/or gray matter with the periventricular white matter being affected in some cases. Peracute lesions consist of foci of hemorrhage and necrosis adjacent to blood vessels. Chronic lesions have a granular yellow-brown to grey appearance. Early microscopic lesions include tachyzoite infection of and proliferation within endothelial cells, leading to ischemia and necrosis of surrounding neuropil15. Later lesions are characterized by nonsuppurative encephalomyelitis and the presence of tachyzoites and tissue cysts in neurons and neuropil with the amount of necrosis, gliosis, neovascularization and white matter injury dependent on the duration of the lesion1. More chronic lesions contain prominent lymphocytic and histiocytic perivascular and leptomeningeal aggregates. Over time, due to host immune defense mechanisms tachyzoites change to bradyzoites that replicate more slowly and form tissue cysts15.
Diagnosis of neosporosis involves histological detection of lesions, immunohistochemistry illustrating tachyzoite and bradyzoite phases, PCR detection of parasite DNA and serology8.
Treatment with corticosteroids is contraindicated in cases of neosporosis11 as immunosuppressed dogs may shed more oocysts than immunocompetent dogs7. In this greyhound, the numerous cysts within the CNS were presumably a consequence of corticosteroid treatment. In studies, dogs that had been given corticosteroids shed more than 100,000 oocysts after being fed with infected murine brains. Various pathological manifestations of neosporosis have been reported in dogs on immunosuppressive therapy, including cerebellar inflammation and atrophy (which can occur in the absence of steroid treatment), the presence of tachyzoites in cerebrospinal fluid and protozoal hepatitis9,10,11. Treatment with currently available drugs including clindamycin is considered only partially effective. None of the currently available drugs are considered capable of killing tissue cysts8.
The predominant cerebrocortical distribution is an unusual neuroanatomic pattern for this infection. We believe that the white matter degeneration within the crus cerebri is secondary following the cerebral cortical necrosis while focal Purkinje cell loss in the midvermis is probably due to brain herniation. While the neocortical inflammation and necrosis in this case is due in large part to the parasites, prolonged seizures may also have contributed to this pattern of necrosis.
JPC Diagnosis: Cerebrum: Meningoencephalitis, necrotizing, segmental, severe with numerous intracellular and extracellular apicomplexan cysts, Greyhound (Canis familiaris), canine.
Conference Comment: The protozoal organisms in the phylum Apicomplexa that result in encephalomyelitis are Toxoplasma, Hammonidia, Sarcocystis, and Neospora. Of the four, Neospora is the most recent addition. First identified in 1988 by Drs. Dubey, Carpenter, and Speer4,6 canine protozoan encephalomyelitis was originally thought to be caused by Toxoplasma.
Toxoplasma gondii which is still an important differential diagnosis (as mentioned above) uses domestic cats and other Felidae as its definitive host, whereas all other warm-blooded animals can act as intermediate host. Interestingly, cats can also act as intermediate hosts where the parasite continues in an extraintestinal cycle. Infection can occur in one of three ways: (1) ingestion of meat containing tissue cysts, (2) ingestion of food contaminated with cat feces which contain sporulated oocysts, and (3) infection in utero. Transplacental infection is most common in sheep, among domestic animals, where infection begins as a primary placentitis affecting predominately the cotyledons with progressive seeding of the organism to the fetus. Late abortions are common with tissue cysts present in the fetal brain and myocardium. Areas of mineralization may accompany cysts in the brain most likely due to hypoxia from placental insufficiency during pregnancy. Following ingestion of oocysts or tissue cysts by intermediate hosts, the organism forms tissue cysts within the CNS, skeletal and heart muscles most commonly. These cysts may survive for the life of the host and can either: (1) become latent and cause no clinical signs or (2) result in acute, necrotizing disseminated infections affecting CNS, lung, myocardium, liver, pancreas, skeletal muscle, and lymph nodes. The organ affected depends mostly on the host. For instance, in cats severe interstitial pneumonia is common, and in adult dogs, concurrent polymyositis and encephalomyelitis is the most common scenario. In puppies and young dogs, however, polyradiculoneuritis is the most common pattern. In the case of latent infections, tissue cysts may show up as incidental findings on necropsy or, if the animal is immune suppressed, may convert to an active infection. There is an association in dogs with Morbillivirus infection and toxoplasmosis, presumably because of the immunosuppressive action of the virus.13
Neospora caninum was identified by Dubey and his colleagues in a retrospective study of 23 cases of canine “toxoplasmosis” in which this new organism was distinguishable from Toxoplasma only by electron microscopy and immunohistochemistry4,6. Ultrastructurally, Neospora tachyzoites lack micropores, but have numerous micronemes and more rhoptries than Toxoplasma gondii. Additionally, Toxoplasma replicates exclusively in parasitophorous vacuoles, whereas, Neospora can use a parasitophorous vacuole or replicate free within the cell cytoplasm3.
Contributing Institution:
The Royal Veterinary College
Hatfield, England
References:
- Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 5th Vol. 2. St. Louis, MO: Elsevier; 2007:272.
- Buxton D, McAllister MM, Dubey JP. The comparative pathogenesis of neosporosis. Trends in Parasitology. 2002;18:546-552.
- Cheville Pathogenic protozoa. In: Ultrastructural Pathology The Comparative Cellular Basis of Disease. 2nd ed. Ames, IA: John Wiley & Sons; 2009:555.
- Dubey JP. A review of Neospora caninum and Neospora-like infections in animals. J Protozool Res. 1992;2:40-52.
- Dubey JP. Review of Neospora caninum and neosporosis in animals. Korean J Parasitol. 2003;41:1-16.
- Dubey JP, Carpenter JL, Speer CA, et al. Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc. 1988;192:1269-1285.
- Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. 2007;20:323-367.
- Dubey JP, Schares G. Neosporosis in animals--the last 5 years. Vet Parasitol. 2011;180:90-108.
- Fry DR, McSporran KD, Harvey C. Protozoal hepatitis associated with immunosuppressive therapy in a dog. J Vet Intern Med. 2009;23:366-368.
- Galgut BI, Janardhan KS, Grondin TM, Harkin KR, Wight-Carter MT. Detection of Neospora caninum tachyzoites in cerebrospinal fluid of a dog following prednisone and cyclosporine therapy. Vet Clin Pathol. 2010;39:386-390.
- Garosi L, Dawson A, Couturier J, Matiasek L, et al. Necrotizing cerebellitis and cerebellar atrophy caused by Neospora caninum infection: magnetic resonance imaging and clinicopathologic findings in seven dogs. J Vet Intern Med. 2010;24:571-578.
- Howe DK, Sibley LD. Comparison of the major antigens of Neospora caninum and Toxoplasma gondii. International Journal for Parasitology. 1999;29:1489-1496.
- Summers BA, Cummings JF, de Lahunta A. Inflammatory diseases of the central nervous system. In: Duncan L, McCandless PJ, eds. Veterinary Neuropathology. Louis, MO: Mosby; 1995:162-169.
- Taylor MA, Coop RL, Wall RL. Parasites of cattle. In: Taylor MA, ed. Veterinary Parasitology. 3rd West Sussex, UK: Wiley-Blackwell; 2007:121.
- Zachary JF. Nervous system. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Diseases. 5th St. Louis, MO: Elsevier; 2007:809.