CASE III: 525/20 (JPC 4167685)
Signalment:
21-year-old, male (gelding), Connemara, Equus caballus, equine
History:
Initially presented in July with multiple suspected cutaneous melanomas mainly in the perineal region and along the tail as well as a suspected squamous cell carcinoma of the penis. The pony was initially treated with penile amputation and sheath ablation. Enlarged inguinal lymph nodes were removed. A number of the melanomas were removed at a second surgery. Presented again in November with neurological signs including head pressing and ataxia. Given the rapidly deteriorating clinical condition, the pony was euthanized.
Gross Pathology:
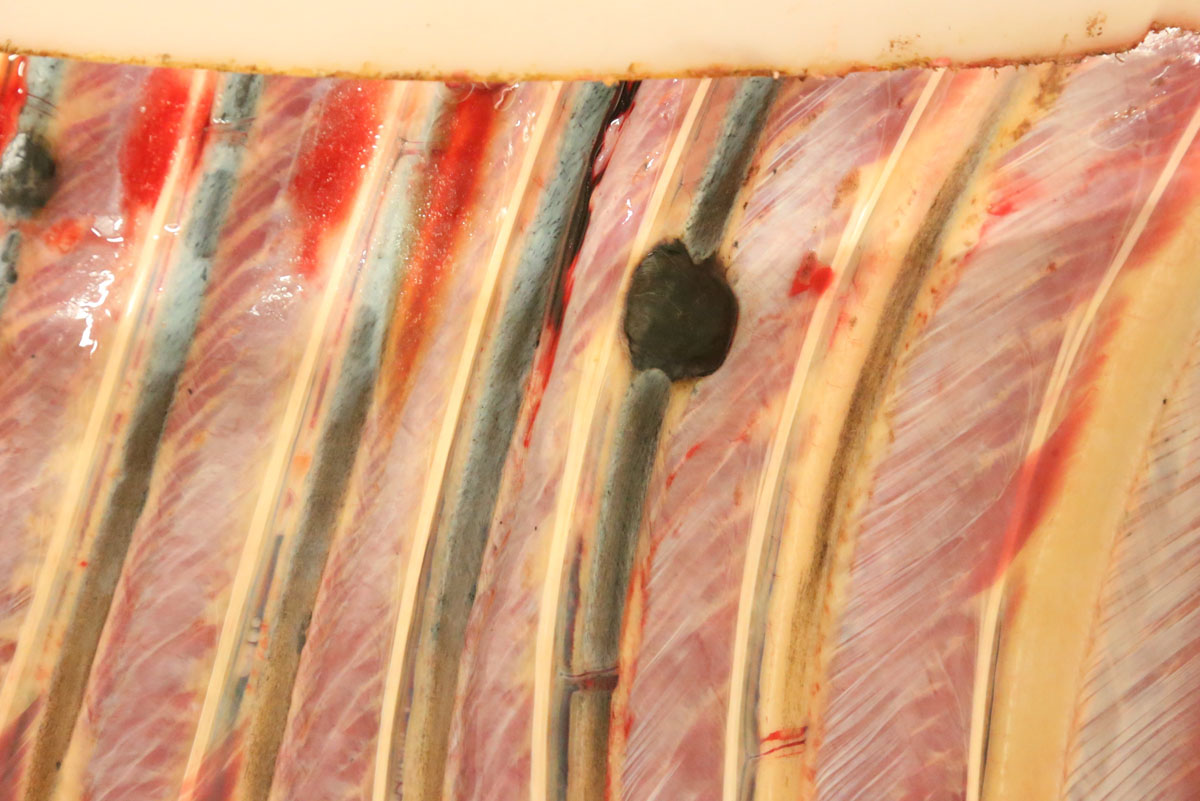
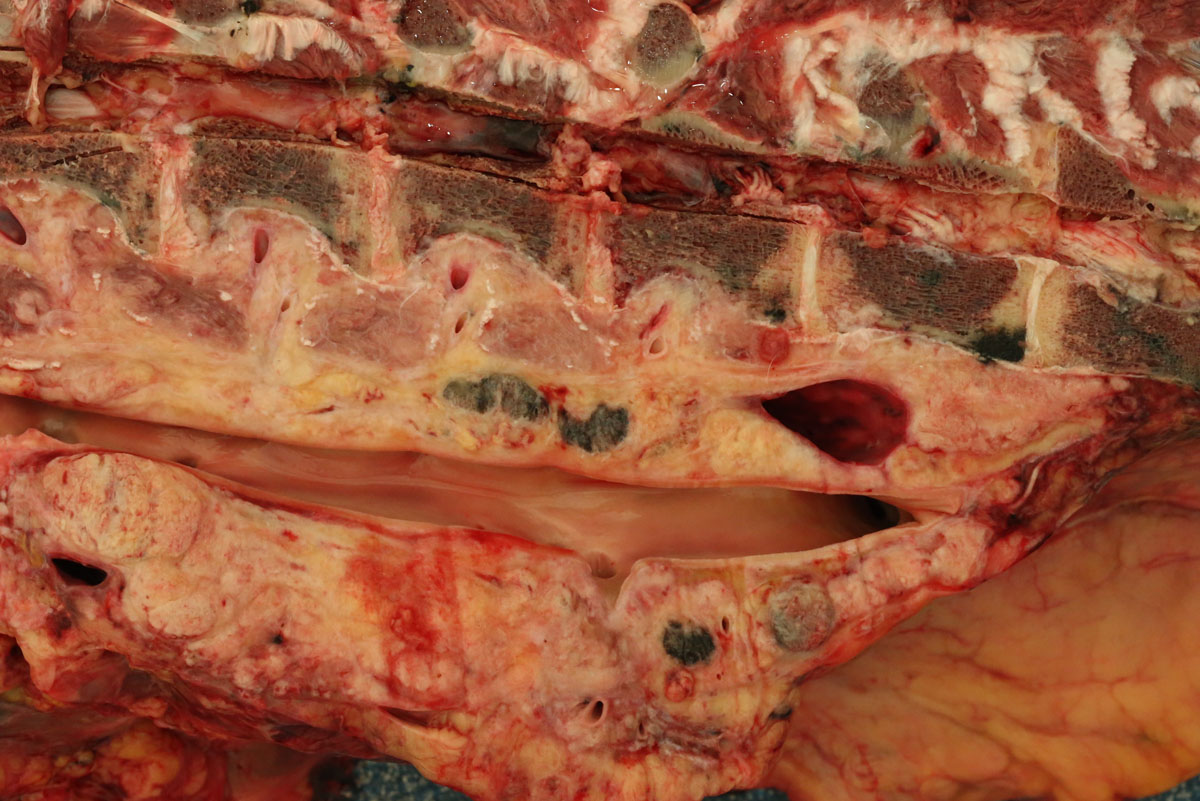
Carcass preservation and body condition were good. Multiple, firm, well-demarcated darkly pigmented dermal masses were observed around the anus and along the tail base. Black pigmentation was observed throughout these masses on sectioning. Further masses of similar appearance and up 6 cm in diameter were found over the ribs, in the region of the brachial plexus, in the dorsal lumbar and gluteal fascia and in the tensor fascia latae muscle. Masses were focally embedded on the pleural aspect of the ribs: approximately 0.5 cm thick and extending to the width of the affected rib. The deep inguinal lymph node was markedly enlarged and subtotally effaced by yellow firm irregular masses. A firm pale yellow lesion infiltrated the psoas muscles and iliac lymph nodes and surrounded the aorta, the ureter and the renal hilus over a distance of some 50cm. This mass was 30cm wide and 15cm deep and abutted the lumbar vertebrae. On sagittal sectioning the lumbar spinal column multiple small pigmented masses (melanomas) were noted embedded within the pale yellow mass. Similar pigmented masses infiltrated numerous lumbar vertebral bodies. On sagittal sectioning of the skull a 5 cm diameter pigmented mass infiltrated the sphenoid bone midline at the level of the pituitary fossa and protruded approximately 1 cm into the cerebral cavity.
Laboratory Results:
None submitted.
Microscopic Description:
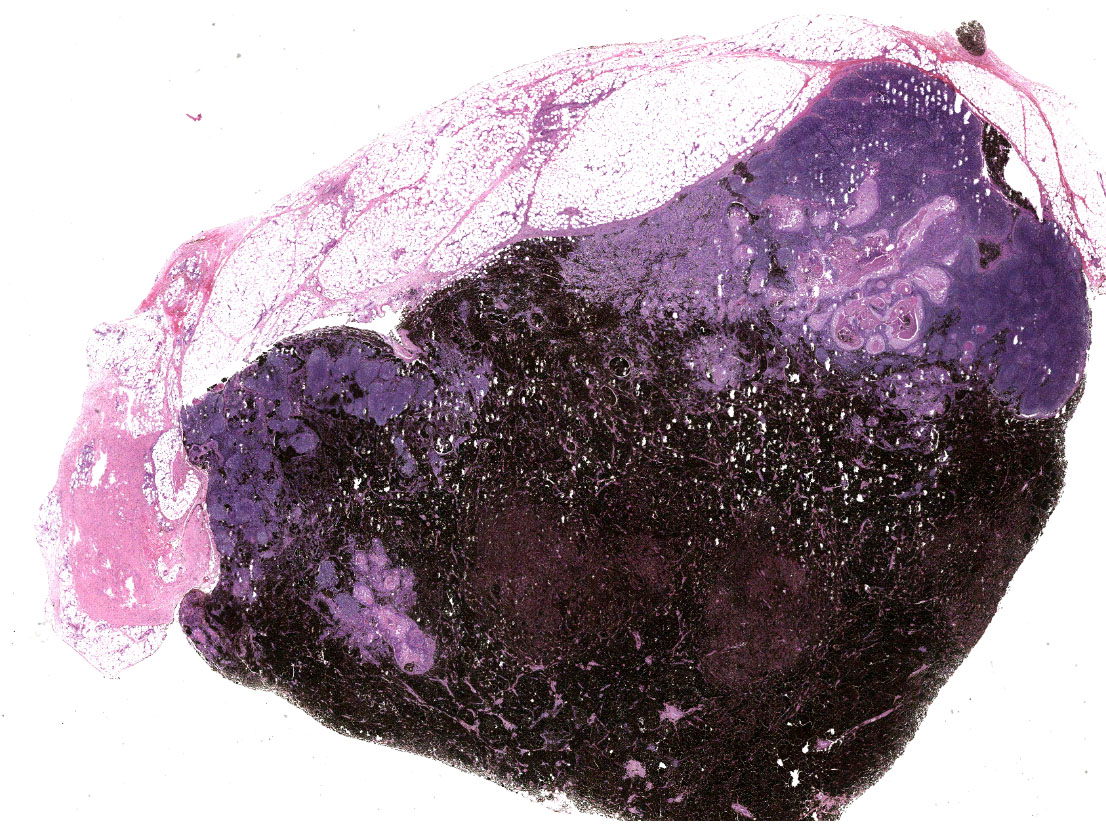
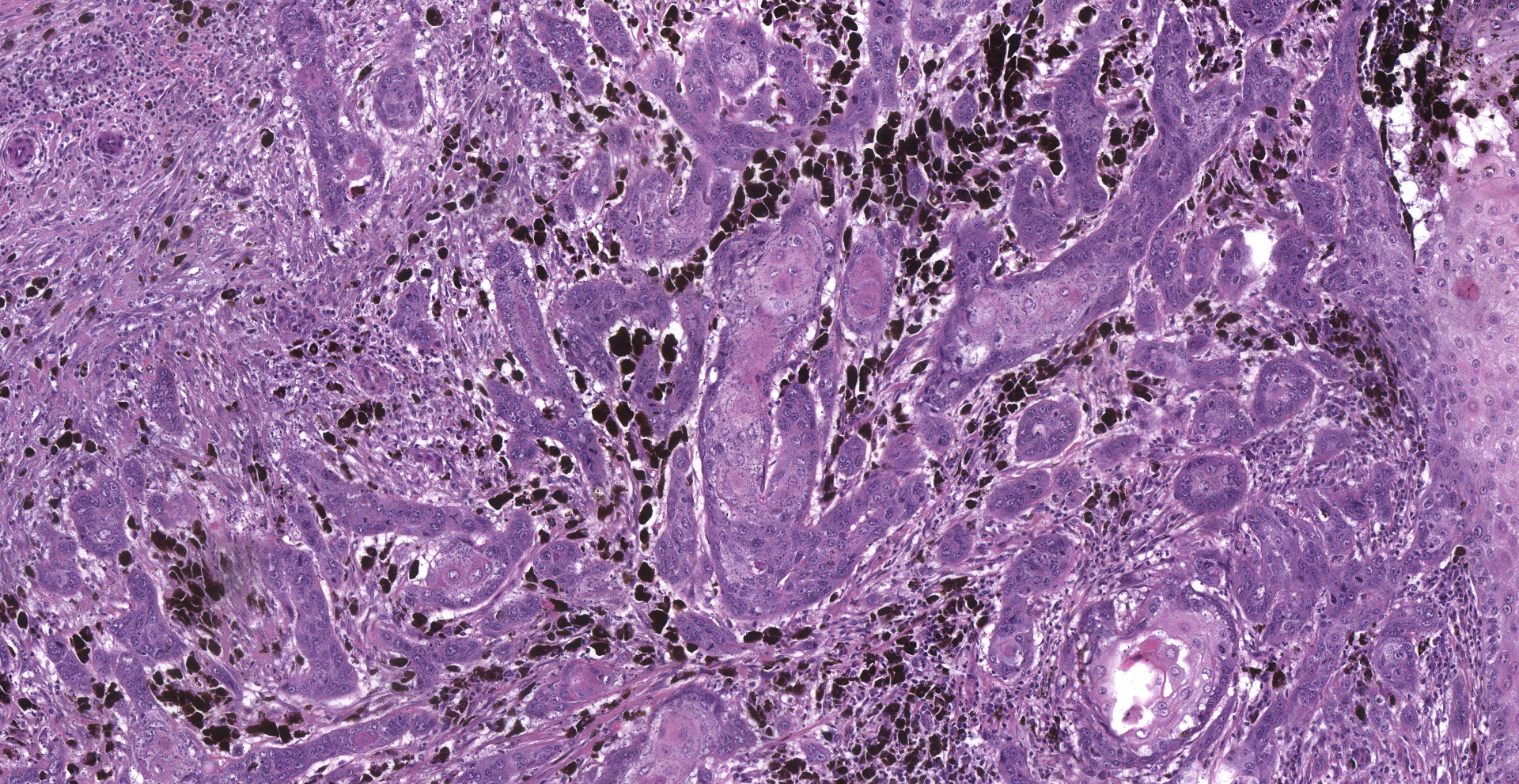
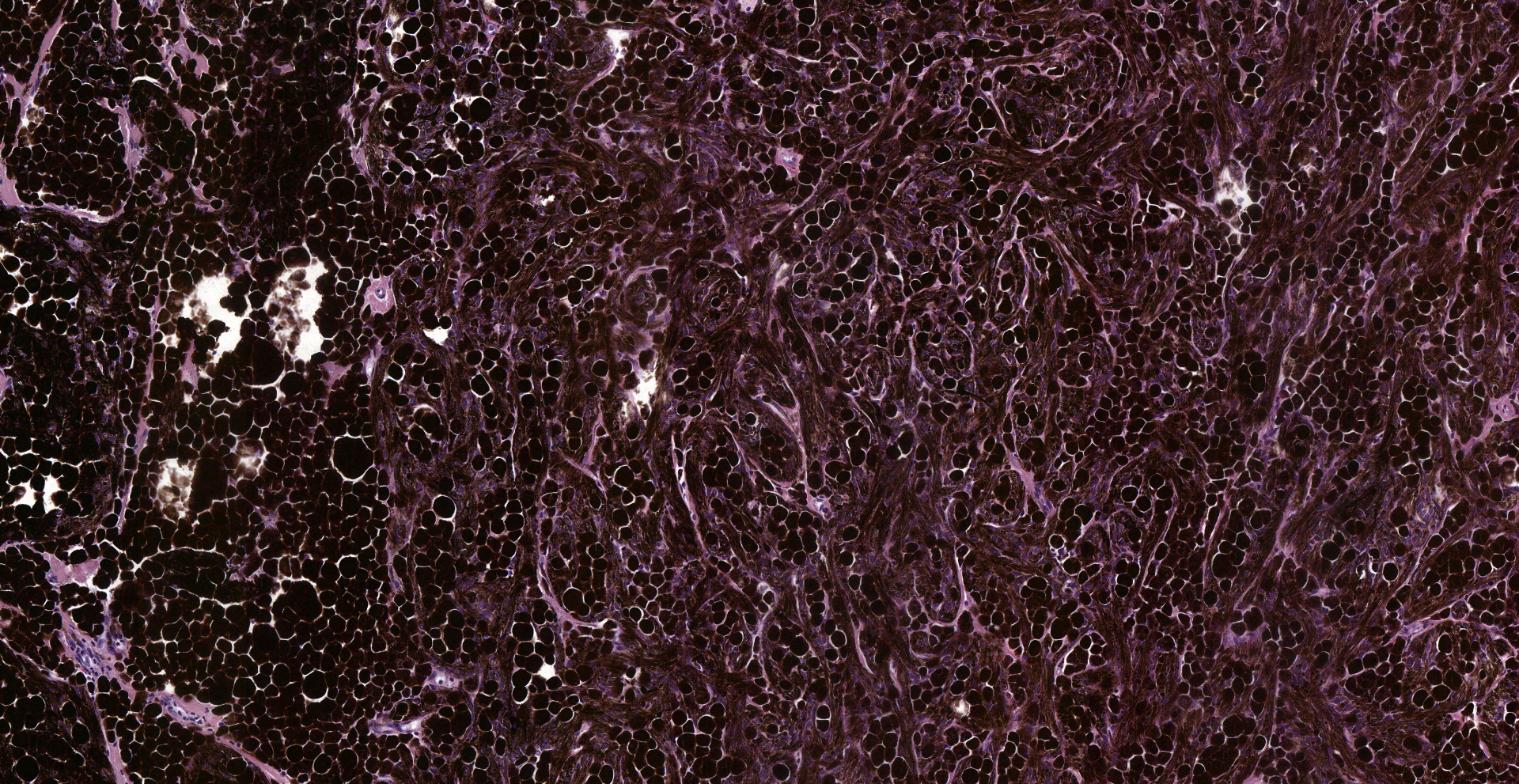
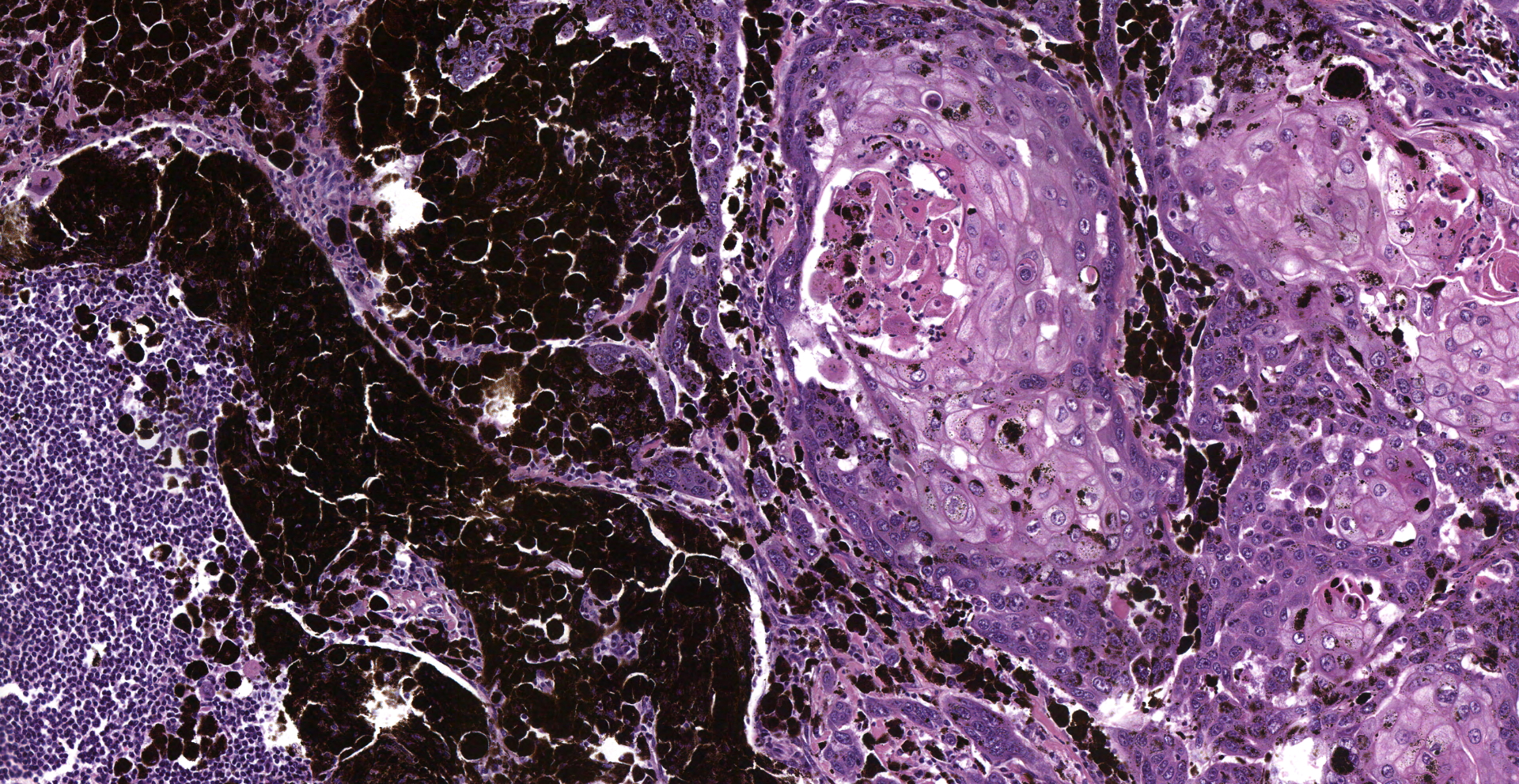
Inguinal lymph node: sub-totally effaced by a poorly demarcated densely cellular mass that infiltrates the surrounding capsule and adipose tissue. The mass is composed of nests and islands of large polygonal cells in a fibrovascular stroma. The majority of the cells have distinct borders and moderate to abundant eosinophilic cytoplasm. The nucleus is either relatively large and ovoid with coarse stippled chromatin or vesicular with a prominent nucleolus. There is marked anisocytosis and anisokaryosis. More than 40 mitotic figures are counted in 10 high power fields. Multifocally the neoplastic islands show areas of central necrosis. In addition to this neoplastic lesion there is a second neoplastic cell population within the node. This consists of more loosely arranged large polygonal cells with distinct cell borders and moderate to abundant cytoplasm featuring myriad large brown granules (melanin). Nuclear details are obliterated by the dense cytoplasmic pigment. Pigmented polygonal cells are occasionally admixed with more spindled cells that contain lesser amounts of cytoplasmic granulation and have a more elongated nucleus. Clustered neoplastic pigmented cells noted in subcapsular sinus.
Histopathology confirmed that the penile squamous cell carcinoma had also metastasized to the iliac lymph nodes and had severely infiltrated the psoas muscles and retroperitoneal region. The melanoma had metastasized widely: skeletal muscles, lymph nodes, fascia, peripheral nerves and bones of the vertebral column and the skull
Contributor's Morphologic Diagnoses:
Inguinal lymph node:
(1) Squamous cell carcinoma
(2) Melanoma
Contributor's Comment:
These 'two for the price of one' diagnoses showcase two 'classic' equine tumors occurring contemporaneously in the same lymph node as a result of metastasis from different anatomical sites.
Between 4 and 15% of all equine skin tumors are melanocytic.6 Melanoma is a result of uncontrolled proliferation of melanocytes that can arise within the epidermis but can also originate from mucosal surfaces and the eye. Although the vast majority of cutaneous melanomas in the horse are benign at initial presentation, over 60% can progress to malignancy with attendant widespread metastasis.6 As in this case, the majority of melanocytic tumors occur in grey or white horses, usually at or before the age of 5 years: the time at which they undergo changes in coat colour.9 A retrospective study of the pathological features of 53 cases of equine melanoma proposed four categories of tumor: melanocytic naevus; discrete dermal melanoma (benign and malignant forms); dermal melanomatosis; and anaplastic malignant melanoma.13 Equine melanomas result from the activation of oncogenes (e.g. NRAS) or from the loss of tumor suppressor genes (e.g. TP53).18 In contrast to melanomas in other/non-grey horses, which are highly malignant, those in grey horses have a longer benign growth phase prior to malignant transformation and metastasis.6,10 Up to 80% of grey horses over 15 years old have melanocytic tumors at some anatomical location.16
The STX17 (syntaxin 17) gene is central to the inheritance of skin pigmentation in grey horses. Both this and the adjacent NR4A3 gene are overexpressed in melanomas from grey horses: elevated STX17 expression is associated with activation of the extracellular signal-regulated kinase (ERK) pathway in melanocytes.5 Increased signaling of the melanocortin-1 receptor (MC1R) gene, a gene that regulates skin pigmentation, also promotes melanoma development in grey horses, as in human patients.16
As in this case, equine melanomas occur most frequently on the perineum/ventral tail region, as well as on the vulva, lips, and eyelids.3,9 One unifying feature of these locations is their adjacency to mucosal epithelium. Equine melanomas are typically positive for S100, PCNA, Ki-67, and CD44 on immunohistochemistry.13,14 In addition, RACK1, has also been found to be effective in distinguishing benign melanocytic tumors from melanomas in horses.1
After sarcoids, squamous cell carcinomas (SCC) are the most common equine neoplasm.14 Around 10% of all equine neoplasms are found on the penis, vulva and ocular adnexa, of which penile SCC is the most common15: between 50?80% of all tumors of the external genitalia. While there is no breed predisposition, a higher incidence of penile SCC is reported in ponies relative to horses.7 Although SCC can develop at any location on the skin surface, like melanoma, it is over-represented on regions contiguous to mucocutaneous junctions. Horses are typically diagnosed with SCC between the ages of 9 and 15 years.7 Risk factors associated with the development of SCC in horses include lightly pigmented skin and chronic exposure to excessive sunlight. It is suspected that SCC frequently develops from pre-existing lesions such as actinic keratosis, carcinoma-in-situ, and the chronic keratosis associated with exposure to accumulated smegma.7 As in human patients, evidence suggests cutaneous SCC in horses result from mutations in the p53 tumor suppressor gene.11 Other research points to oncogenesis being initiated, in part, by equine papillomavirus type 2-infection, similar to the sexually-transmitted infection model proposed for cervical cancer in human patients.2,17,19
Most SCC in horses arise as solitary masses but the adjacent skin may be at similar risk of tumor development, particularly the prepuce.6 Cutaneous SCC are locally invasive, metastasising to local lymph nodes in some 19% of cases (as described here). These tumors can ulcerate or become traumatized leading to secondary bacterial infections and purulent inflammation.
Contributing Institution:
Room 012, Veterinary Sciences Centre, School of Veterinary Medicine, University College Dublin, Belfield, Dublin 4, Ireland
http://www.ucd.ie/vetmed/
JPC
Diagnosis:
1. Lymph node: Metastatic squamous cell carcinoma.
2. Lymph node: Metastatic melanoma.
JPC Comment:
We would like to thank the contributor for this fantastic submission of two classic equine neoplastic entities in one tissue section. In addition, the contributor concisely highlights key features of each entity.
As noted by the contributor, both graying and melanoma in horses have been linked to a duplication of the syntaxin-17 (STX17) gene. The significance of this duplication is the STX17 gene plays a regulatory role in the regulatory binding sites for micropththalmia-associated transcription factor (MITF) and NR4A3, both of which play key roles regulating melanocyte gene expression and cellular function. Horses homozygous for the STX17 duplication have two additional copies of this regulatory element and have a higher incidence of melanoma as well as a higher mean melanoma grade in comparison with heterozygous grey horses.12
An additional mutation associated with increased melanoma severity is the deletion
of exon 2 in the agouti-signaling protein (ASIP) gene, which regulates the melanocortin-1-receptor (MC1R) pathway. NR4A3 and MITF transcription is dependent on the MC1R pathway and cause an increase in the cellular concentration of cAMP. Under normal conditions, the MC1R signaling pathway is inhibited (i.e. regulated) by agouti-signaling protein, which in turn decreases cAMP, MITF, and NR4A3 expression. Horses homozygous for the deletion of the exon 2 of the ASIP gene are unable to antagonize the MC1R pathway, resulting in increased signaling and has been associated with increased melanoma severity in horses.12
In contrast, "chestnut" horses occur as the result of a loss-of-function mutation of the MC1R gene, resulting in reduced melanocyte cAMP levels and decreased MITF and NR4A3 transcription. In humans, similar loss of function mutations in the MC1R gene do not decrease the risk of developing melanoma but are associated with better survival rates. Although the MC1R loss of function mutation may be associated with similar outcomes in horses, this phenomenon has yet to be definitively validated in this species.12
Horses are affected by nine types of Equus caballus papillomavirus (EcPV), which are non-enveloped double stranded DNA viruses. As noted by the contributor, EcPV-2 has been associated with genital lesions and that in some cases undergo neoplastic transformation to squamous cell carcinoma. Potential ongogenic mechanisms have not yet been definitively determined. However, genomic sequencing has shown the presence of both E6 and E7 genes.4 In contrast to high risk-human paplillomaviruses (hr-HPVs) which are known to cause cervical cancer, EcPV-2 E7 lacks the RB-binding motif. Therefore, the primary oncogene of interest EcPV-2's is E6, which in hr-HPVs inactivates p53 by signaling for its proteosomal degradation.4 Also known as "the guardian of the genome", p53 is a tumor suppressor gene that regulates the cell cycle, DNA repair, cellular senescence, and apoptosis. Loss of p53 function results in the accumulation of damaged (i.e. unrepaired) DNA resulting in the accumulation of driver mutations in oncogenes that ultimately predispose the cell to malignant transformation.8
References:
1. Campagne C, Julé S, Bernex F, Estrada M, Aubin-Houzelstein G, Panthier J-J, Egidy G. RACK1, a clue to the diagnosis of cutaneous melanomas in horses. BMC Vet Res. 2012; 8: 95.
2. Elce, Y. The aetiopathogenesis of squamous cell carcinomas in horses. Where are we? Equine Vet Educ. 2009; 21:17?18
3. Fleury C, Berard F, Balme B, Thomas L. The study of cutaneous melanomas in Camargue-type gray-skinned horses (1): clinical-pathological characterization. Pigment Cell Res. 2000;13: 39?46.
4. Greenwood S, Chow-Lockerbie B, Epp T, et al. Prevalence and Prognostic Impact of Equus caballus Papillomavirus Type 2 Infection in Equine Squamous Cell Carcinomas in Western Canadian Horses. Vet Pathol. 2020;57(5):623-631.
5. Jiang L, Campagne C, Sundström E, et al. Constitutive activation of the ERK pathway in melanoma and skin melanocytes in grey horses. BMC Cancer. 2014;14:857.
6. Johnson PJ. Dermatologic tumors (excluding sarcoids). Vet Clin North Am Equine Pract. 1998;14: 625?658.
7. Knowles EJ, Tremaine WH, Pearson GR, Mair TS. A database survey of equine tumours in the United Kingdom. Equine Vet J. 2016:48; 280?284.
8. Kumar V, et al. Neoplasia. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran: Pathologic basis of disease. 10th ed. Philadelphia, PA: Elsevier; 2021: 294-297.
9. Seltenhammer MH, Heere-Ress E, Brandt S, et al. Comparative histopathology of grey-horse-melanoma and human malignant melanoma. Pigment Cell Res. 2004;17: 674?681.
10. Schöniger S, Summers BA. Equine skin tumours in 20 horses resembling three variants of human melanocytic naevi. Vet Dermatol. 2009; 20: 165?173
11. Teifke P, Lohr CV. Immunohistochemical detection of p53 overexpression in paraffin wax-embedded squamous cell carcinomas of cattle, horses, cats and dogs. J. Comp. Path. 1996; 114: 205-210
12. Teixeira RB, Rendahl AK, Anderson SM, et al. Coat color genotypes and risk and severity of melanoma in gray quarter horses. J Vet Intern Med. 2013;27(5):1201-1208.
13. Valentine BA. Equine melanocytic tumors: a retrospective study of 53 horses (1988 to 1991). J Vet Intern Med. 1995; 9: 291?297.
14. Valentine BA. Survey of equine cutaneous neoplasia in the Pacific Northwest. J Vet Diagn Invest. 2006:18; 123?126.
15. Van den Top JGB, Ensink JM, Grone A, Klein WR, Barneweld A, Van Weeren PR. Penile and preputial tumours in the horse: Literature review and proposal of a standardised approach. Equine Vet J. 2010; 42: 746?757
16. Van der Weyden L, Brenn T, Patton EE, Wood GA, Adams DJ. Spontaneously occurring melanoma in animals and their relevance to human melanoma. J Pathol. 2020; 252: 4?21
17. Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJF, Peto J, Meijer CJLM, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12?19
18. Wong K, van der Weyden L, Schott CR, Foote A, Constantino-Casas F, Smith S, et al. Cross-species genomic landscape comparison of human mucosal melanoma with canine oral and equine melanoma. Nat Commun. 2019;10:353.
19. Zhu, K. W., Afolter, V. K., Gaynor, A. M., Dela Cruz, F. N. & Pesavento, P. A. Equine genital squamous cell carcinoma: in situ hybridization identifies a distinct subset containing equus caballus papillomavirus 2. Vet Pathol. 2015; 52:1067?1072.