CASE 1: S17/4906 (4116732-00)
Signalment: 4.75Y, female, Montbéliarde, Bos taurus, bovine
History: On a dairy farm in the canton of Jura in northwestern Switzerland, four cows presented with a history of persistent fever over several days. Two to three weeks prior, another cow from the same farm suddenly died without noticeable premortal symptoms. A field necropsy conducted on this cow revealed nodular hemorrhagic lesions in the lungs, which tested positive for Bacillus anthracis. The four cows with fever repeatedly tested negative for B. anthracis via blood culture. Three of these four cows spontaneously recovered; the fourth cow showed signs of an incipient abortion and was treated once with penicillin. The next day, the veterinary authorities decided to submit the cow for necropsy to elucidate the cause of this nonspecific feverish illness. Sixteen hours after treatment, the cow was transported alive to the pathology laboratory for diagnostic testing, euthanasia, and necropsy under special biosafety measures.
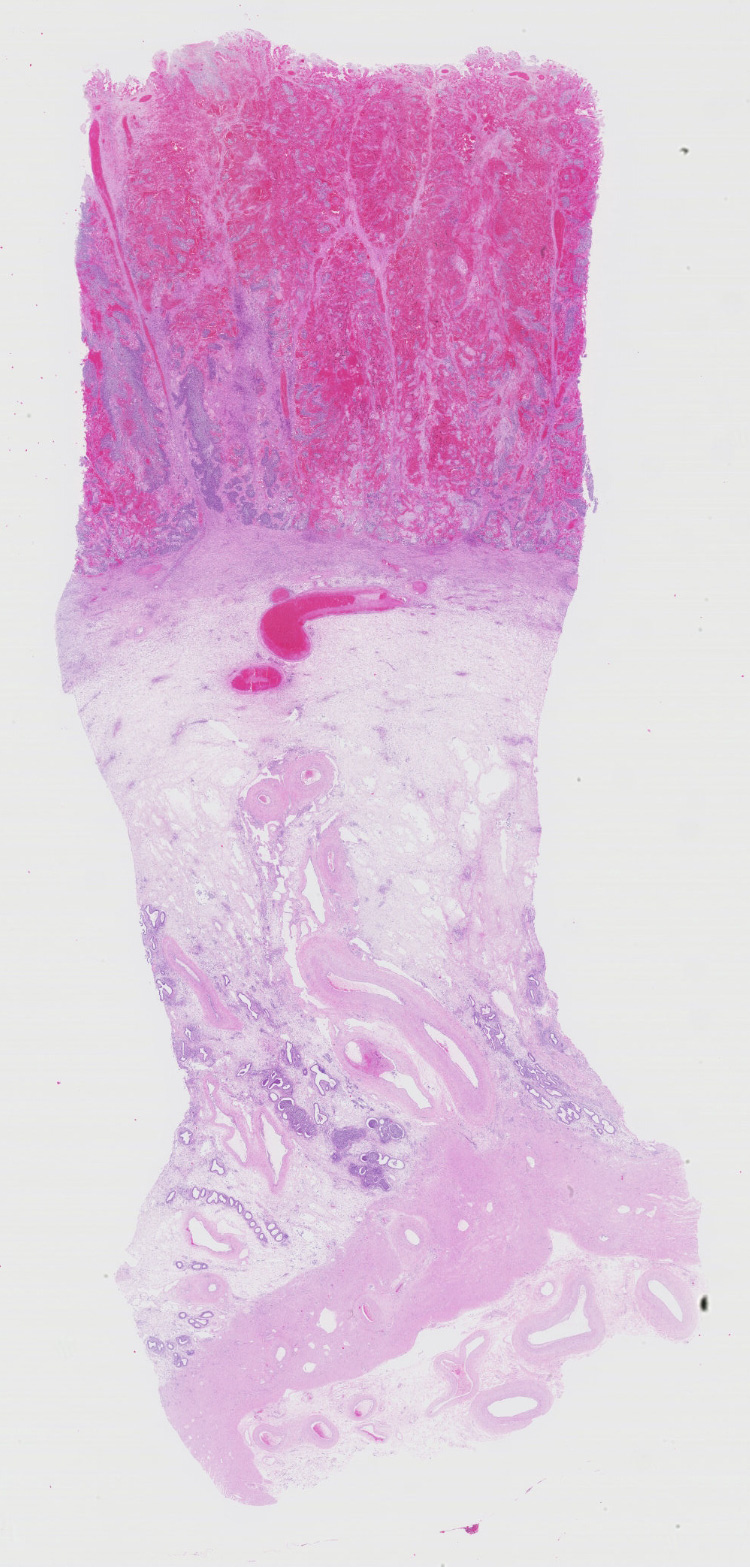
Gross Pathology: A small amount of red-tinged, viscous vaginal discharge was present. Further examination demonstrated marked subcutaneous edema of the ventral abdomen, in addition to significant enlargement of the iliac lymph nodes. Examination of the uterus yielded an edematous, expired fetus in blood-tinged amniotic fluid. The placentomes were moderately hemorrhagic and friable and the placenta was easily detachable. The remainder of the necropsy, including the spleen, was grossly unremarkable.
Laboratory results: Giemsa-stained smears and bacterial cultures from blood, spleen, liver and kidney were negative, but antibiotic residues were identified. B. anthracis was isolated culturally in large and small amounts from placenta and iliac lymph node, respectively. The presence of B. anthracis in placental tissue and iliac lymph node was confirmed with a gamma-phage lysis assay and PCR specific for B. anthracis.
Microscopic description:
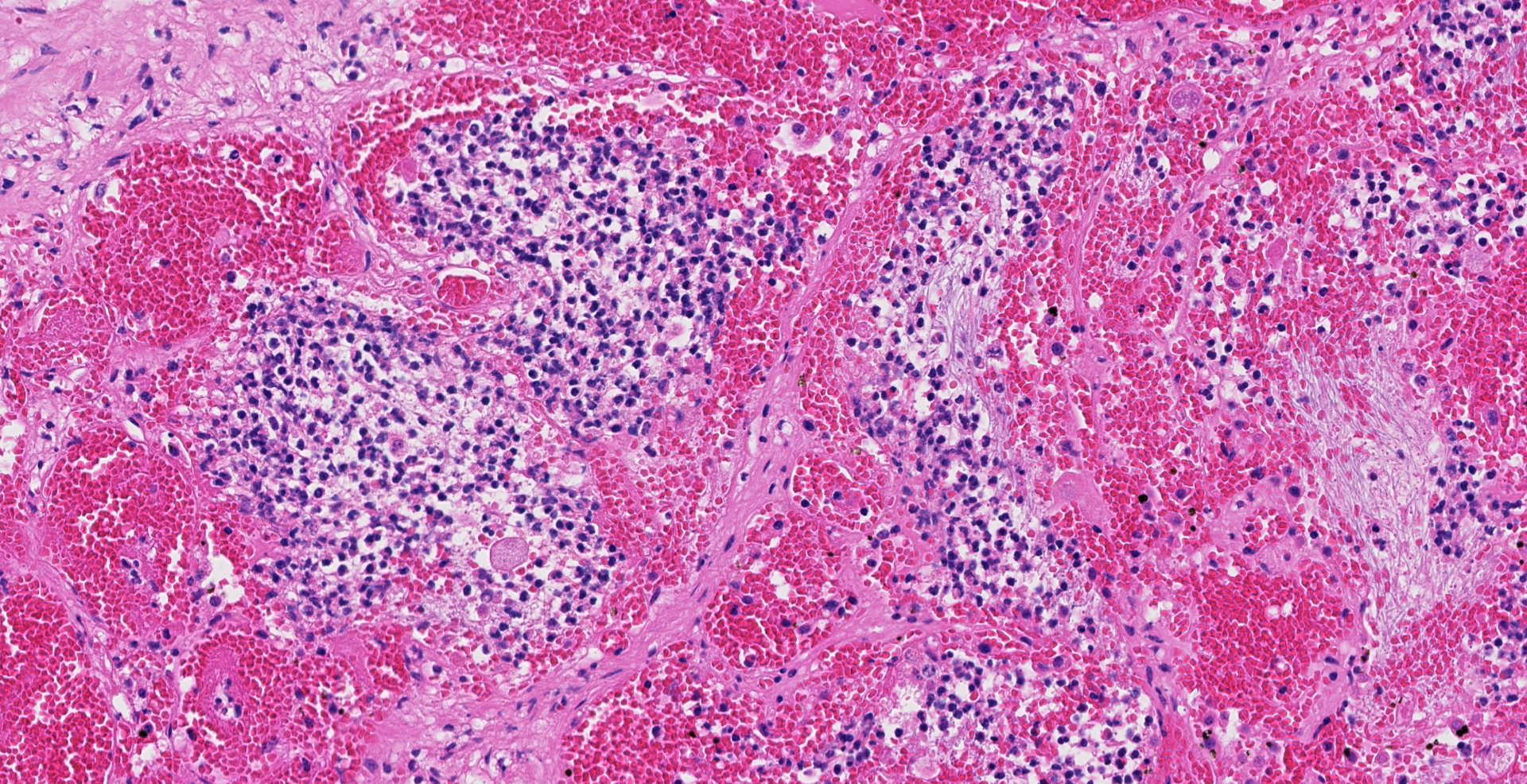
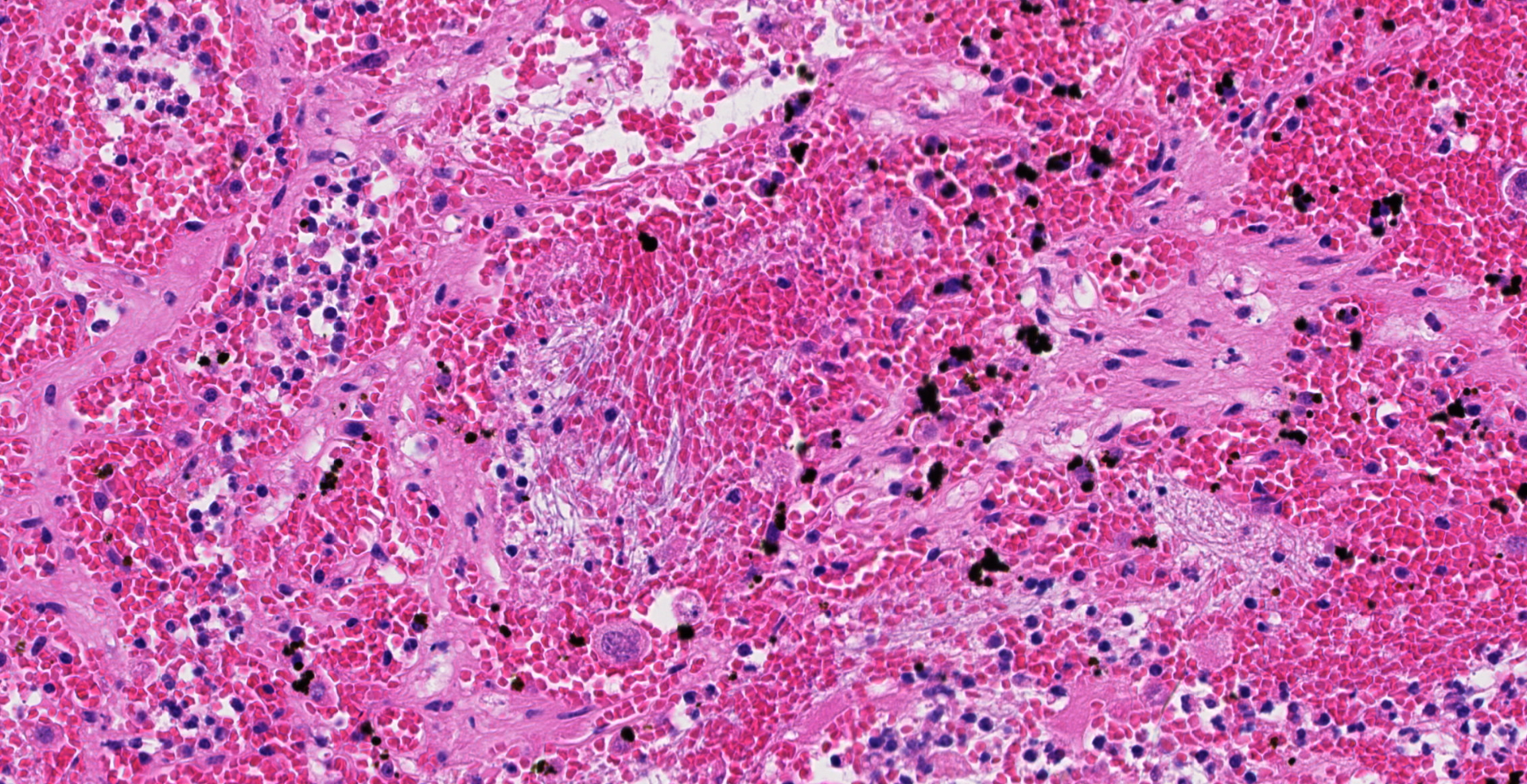
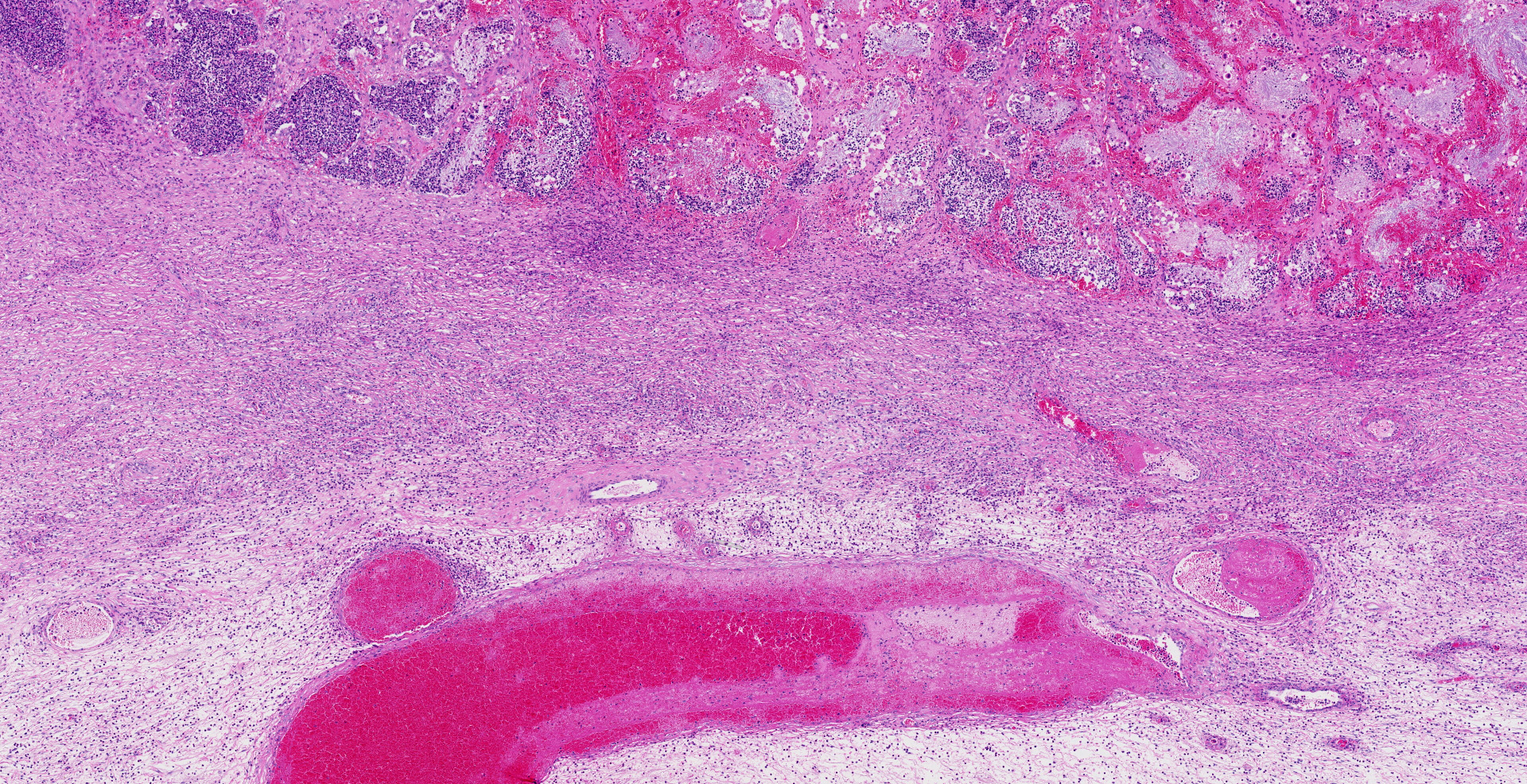
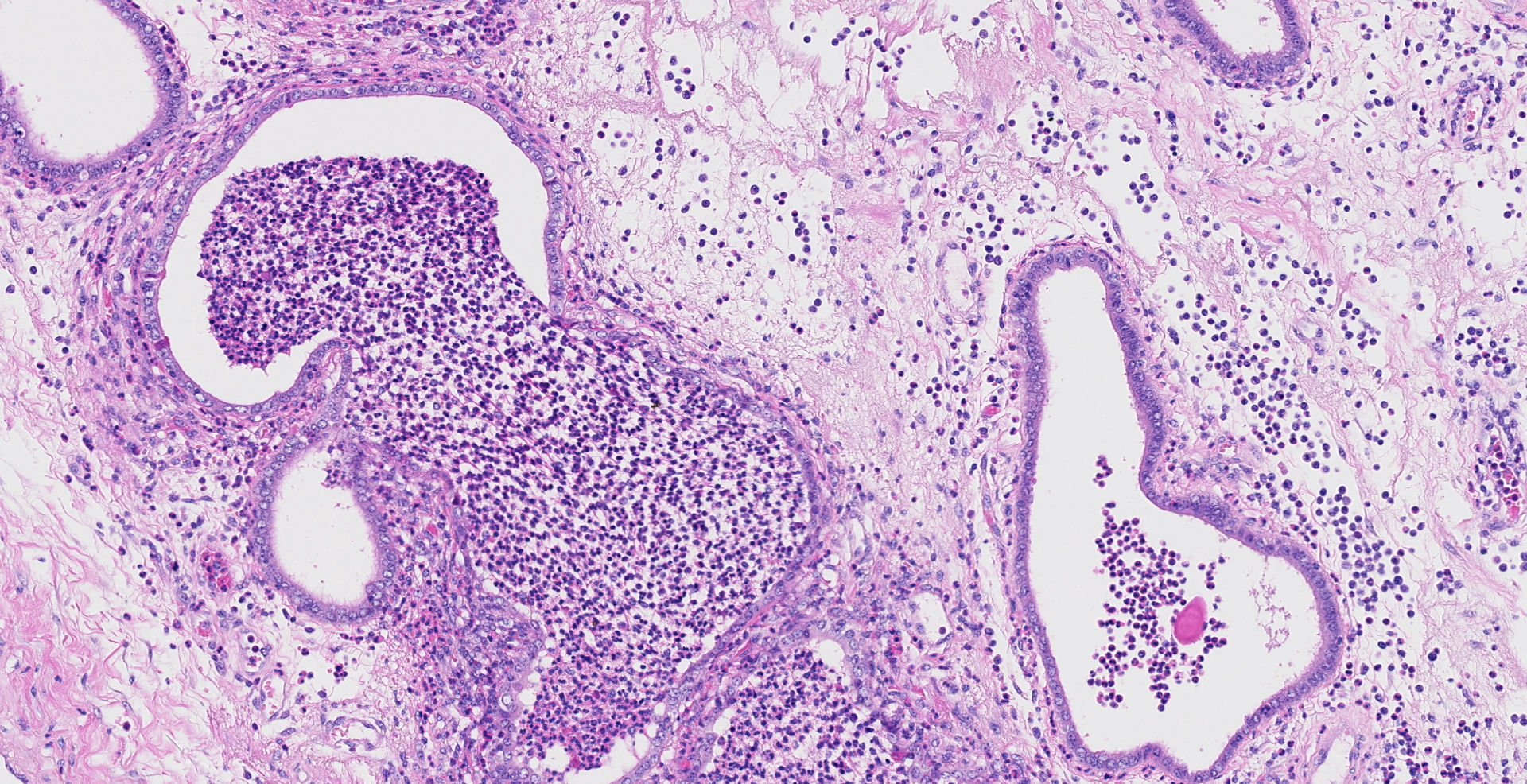
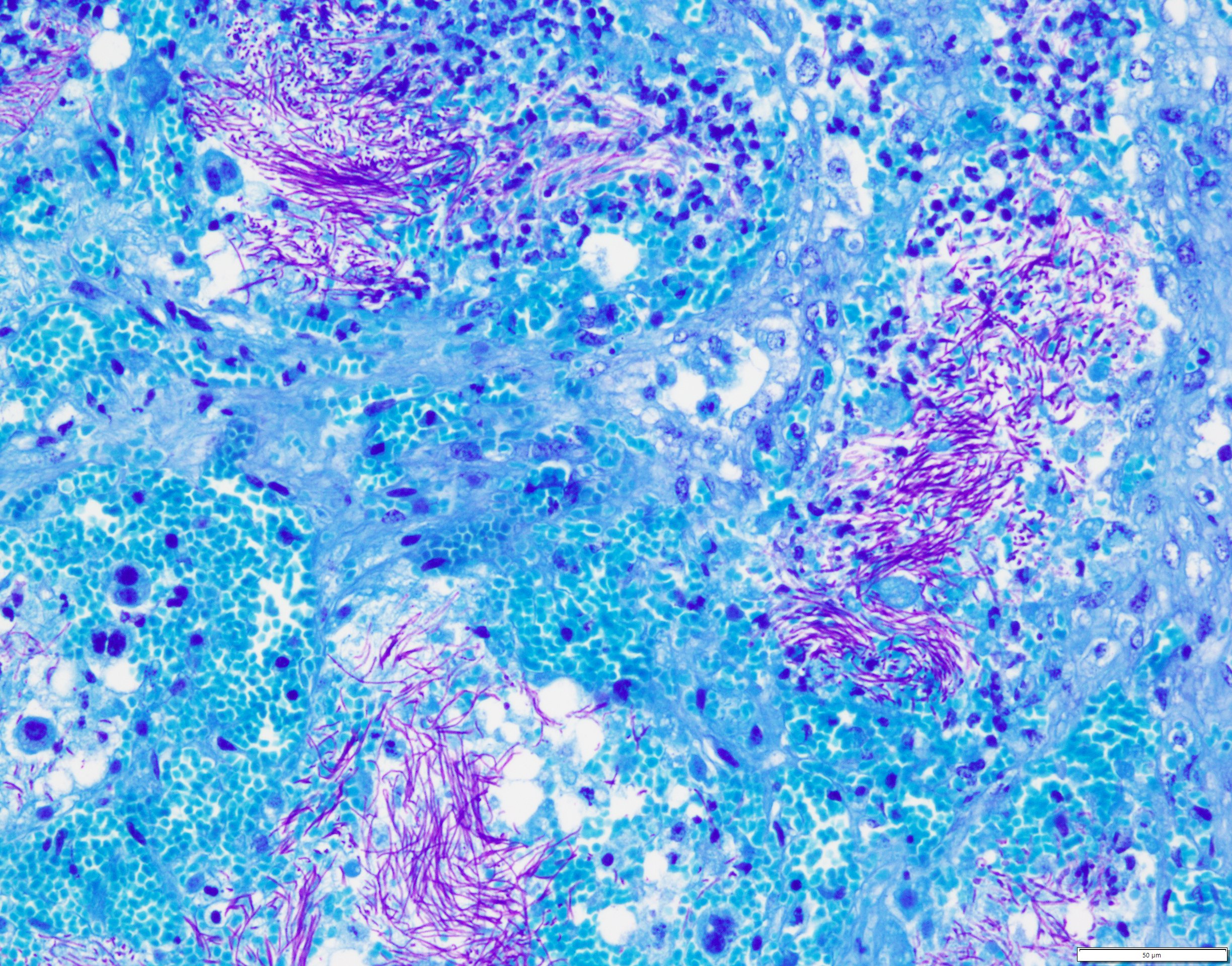
Uterus, placentome: The placentomal tissue shows diffuse and extensive acute hemorrhage and coagulative necrosis affecting both fetal cotyledonary and maternal caruncular parts. Remaining epithelial cells are commonly swollen and pale (hydropic degeneration). The tissue is multifocally infiltrated by moderate to large numbers of mostly degenerated neutrophils. Within the necrosis and hemorrhage, there are numerous long, pale basophilic bacilli evident, often arranged in heaps. The transition zone to the endometrium is heavily infiltrated by degenerated neutrophils. Vessels in this area as well as in the subjacent endometrium exhibit fibrinoid necrosis and neutrophilic infiltration of their wall, associated with perivascular fibrin leakage. The endometrium presents with a massive edema in the lamina propria. Lymph vessels are severely dilated, show loss of endothelial cells, and contain few degenerated neutrophils within their lumina. The endometrial interstitium and the endometrial glands are multifocally infiltrated by numerous mostly degenerated neutrophils.
Gram stain (not submitted): The bacilli are gram-positive.
Iliac lymph node (not submitted): The lymphatic tissue and the adjacent perinodal fatty tissue shows extensive necrosis and infiltration with degenerated neutrophils, associated with extensive edema and fibrinoid vasculitis. In contrast to the placentome tissue, only few gram-positive rod-shaped bacteria are evident.
Liver (not submitted): Multifocal randomly distributed small foci of necrosis and neutrophilic infiltration are evident, consistent with an embolic hepatitis.
Contributor's morphologic diagnosis:
Uterus: Endometritis and placentitis, hemorrhagic, necrotizing and suppurative, severe, diffuse, acute, with fibrinoid vasculitis, severe edema and numerous bacilli.
Contributor's comment: The postmortem and histological findings in combination with the laboratory results are compatible with a localized, non-septicemic form of anthrax. This worldwide occurring zoonotic and usually fatal disease is caused by the Gram-positive, large rod-shaped, spore-forming bacterium Bacillus anthracis.2,4-5,9-10 The vegetative form of B. anthracis is almost exclusively found in vital tissues of warm-blooded animals because of its need for a low oxygen pressure.11 Outside a host, B. anthracis forms non-proliferating endospores, which can persist in the environment for decades and are resilient to ultraviolet radiation, dehydration, and temperature extremes, in addition to many methods of disinfection, save for oxidizing agents.2,10 Nevertheless, in oscillating periods of precipitation, endospores may germinate spontaneously into vegetative forms and may proliferate in the environment to a limited extent.10 According to the Centers for Disease Control and Prevention, B. anthracis endospores are commonly found in the soil of agricultural regions of Central and South America, sub-Saharan Africa, central and southwestern Asia, southern and eastern Europe, and the Caribbean.2
Ruminants are highly susceptible to the disease and are usually infected upon grazing endospore-laden pastures. The ingestion of endospores typically causes a peracute, massive bacteremia only a few hours before death.10-11 In more resistant species, such as horses, swine, and carnivores, the disease is decelerated and the infection is usually localized to the area of endospore entry, which is most likely the gastrointestinal tract 11. Less frequently, the localized form may also be seen in ruminants, manifesting as enteric or pulmonary anthrax.11 In non-septicemic anthrax, death is usually a sequela to the extensive tissue damage caused by bacterial exotoxins.10-11
In humans, most common form is cutaneous anthrax acquired by endospore-contaminated wounds.11 Occasional cases of the alimentary form occur after consumption of contaminated and poorly cooked meat. In earlier times, the third form, pulmonary anthrax, was a frequent human disease especially affecting workers of lane-processing and tanning industries, where bacterial endospores easily aerosolized from contaminated skins and wool.10-11
The mechanism by which B. anthracis invade tissues and lymph vessels is poorly understood, but abrasions or wounds in skin and mucosae as a prerequisite have been proposed. 2,4-5,9-10 In the enteric form, spores and/or vegetative forms are able to evade gastric acidity and reach the intestine by means of peristalsis.10 Germination of endospores likely occurs either in the mucus layer of the mucosa or within Peyer's patches after phagocytosis by M cells.4-5,10
The two principal virulence factors of B. anthracis are the polypeptide capsule to evade phagocytosis and the toxin complex causing cell lysis. The latter being an A-B-class toxin consists of the three proteins protective antigen (PA), edema factor (EF), and lethal factor (LF).9-11 PA binds to receptors on the host cell surface, where it is activated by cleaving. Heptamers of activated PA form pores with competitive binding sites for either EF or LF. PA-EF (edema toxin) or PA-LF (lethal toxin) complexes are internalized by endocytosis, followed by release of EF and LF into the cytoplasm.6 EF is an adenylate cyclase causing edema by disturbance of water and ion exchange. LF is a protease causing cell death by interruption of the MAP kinase pathway.4-5,10-11
The typical gross findings of ruminants succumbed to septicemic anthrax comprise rapid autolysis, hemorrhagic discharge from orifices, and an enlarged, dark red, friable spleen.5,10 Histologically, inflammation is usually absent, as animals typically succumb quickly within hours of development of acute bacteremia.5,10 In alimentary anthrax, gross lesions include segmental to diffuse hemorrhagic enteritis with transmural edema and hemorrhage. Mesenteric lymph nodes may also be enlarged, edematous, and hemorrhagic.5,9 Pulmonary anthrax is characterized by hemorrhagic consolidation of portions of the lungs, associated with interstitial and mediastinal edema and mediastinal hemorrhagic lymphadenitis.9 Infected carnivores, horses and swine most commonly present with extensive edema, hemorrhage and swelling in the oropharyngeal region and the neck, or suffer from the enteric form.9 In histology, localized infections exhibit extensive necrosis, edema and hemorrhage associated with fibrinoid vasculitis and the presence of variable numbers of bacilli.9
Abortion as a sequela to anthrax infection may occur as in other illnesses with fever. The presence of B. anthracis in bovine abortion material, however, has been described only once.3 In the present case, an initial nonlethal bacteremia that resolved spontaneously is suspected to have spread to the placenta, which consequently harbored a local bacterial proliferation and ultimately termination of the fetus. Here, the concurrent necrotizing iliac lymphadenitis is consistent with the spread of bacteria to the regional lymph nodes.
The conduction of necropsies on animals with a suspicion of anthrax should be avoided unless special biosafety infrastructure is available to prevent personnel and environmental contamination. In suspicious cases, blood and all sorts of discharge from body orifices should be tested with sensitive methods, such as culture and PCR, for the presence of B. anthracis before opening the carcass, bearing in mind that they may be false negative due to lack of septicemia or antibiotic treatment.1,7 Thus, it is important to still consider anthrax as a differential in cases of necrohemorrhagic enteritis, pharyngitis, lymphadenitis, or red-tinged discharge from body orifices despite negative blood cultures.
Contributing Institution:
Institute of Animal Pathology
Vetsuisse Faculty, University of Bern
122 Laenggassstrasse
P.O.B.
3001-CH Bern
Switzerland
JPC diagnosis:
Uterus and placenta: Placentitis, necro-hemorrhagic and suppurative, diffuse, severe, with placental vasculitis, edema, mild suppurative endometritis, and numerous extracellular bacilli, Montbéliarde, bovine.
JPC comment: The contributor provided a concise and comprehensive review of the clinical and pathologic features of this entity. In addition, B. anthracis is an illustrious member of the Select Agents and Toxins List under the U.S. Federal Select Agent Program (7CFR Part 331, 9 CFR Part 121, and 42 CFR Part 73), administered jointly by the U.S. Department of Health and Human Services, the U.S. Department of Agriculture, and the Center for Disease Control and Prevention. In fact, B. anthracis is categorized as a Tier 1 agent, along with Ebola virus, smallpox virus, Yersinia pestis, and others. This categorization necessitates additional considerations with respect to research circumstances (facility registration, control, disposal, etc), but diagnostic labs are largely exempt from these requirements. If a specimen is presented, and is diagnosed with a select agent, the facility is expected to: 1) officially transfer the specimen, or destroy the specimen on-site by a recognized sterilization or inactivation process within 7 days, 2) until transfer or destruction, the agent or toxin is secured against theft, loss, or release, 3) original specimens containing the select agent or toxin are transferred or destroyed within 7 days, and 4) the identification of the select agent or toxin is reported to USDA APHIS or CDC.
The main FDA approved vaccine (Biothrax), is made from cell-free filtrates of microaerophilic cultures of an avirulent, nonencapsulated strain of B. anthracis. CDC currently recommends vaccination of certain laboratory workers who work with Bacillus anthracis, people who handle potentially infected animals or their carcasses, some military personnel, and some emergency and other responders whose response may lead to exposure. Additional research continues, and Protective Antigen domain 4 and the combination of nanoparticles and bacteriophage T4 have been recent targets for vaccine development.8,12
References:
1. Brown ER, Cherry WB. Specific identification of Bacillus anthracis by means of a variant bacteriophage. J Infect Dis. 1955;96(1):34-39.
2. Centers for Disease Control and Prevention. Anthrax. http://www.cdc.gov/anthrax/index.html. May 15, 2018.
3. Gibbons DF. Isolation of Bacillus anthracis from an aborted bovine foetus. Nature. 1974;252:612.
4. Glomski IJ, Piris-Gimenez A, Huerre M, Mock M, Goossens PL. Primary involvement of pharynx and Peyer's patch in inhalational and intestinal anthrax. PLoS Pathog. 2007;3(6) :0699-0708.
5. Liu S, Mahtab M, Leppla SH. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol. 2014;22(6):317-325.
6. Petosa C, Collier RJ, Klimpel KR, Leppla SH, Liddington RC. Crystal structure of anthrax toxin protective antigen. Nature. 1997;385:833-838.
7. Ryu C, Lee K, Yoo C, Seong WK, Oh HB. Sensitive and rapid quantitative detection of anthrax spores isolated from soil samples by real-time PCR. Microbiol Immunol. 2003;47(10):693-699.
8. Tao P, Mahalingam M, Zhu J, et al. A bacteriophage T4 nanoparticle-based dual vaccine against Anthrax and Plague. Therapeutics and Prevention. 2018; 9(5): e01926-18.
9. Twenhafel NA. Pathology of Inhalational Anthrax Animal Models. Vet Pathol. 2010;47(5):819-830.
10. Valli VEO, Kiupel M, Bienzle D. Hematopoietic System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. St. Louis, MO; 2016:102-268.
11. WHO, FAOUN, OIE. Anthrax in Humans and Animals. 4th ed. (Turnbull P, ed.). Geneva, CH: World Health Organisation; 2008. doi:10.2105/AJPH.30.3.299.
12. Zakowska D, Graniak G, Rutyna P, Naylor K, Glowacka P, Niemcewicz M. Protective antigen domain 4 of Bacillus anthracis as a candidate for use as vaccine for anthrax. Annals of Agricultural and Environmental Medicine. 2019; 26(3): 392-395.