Signalment:
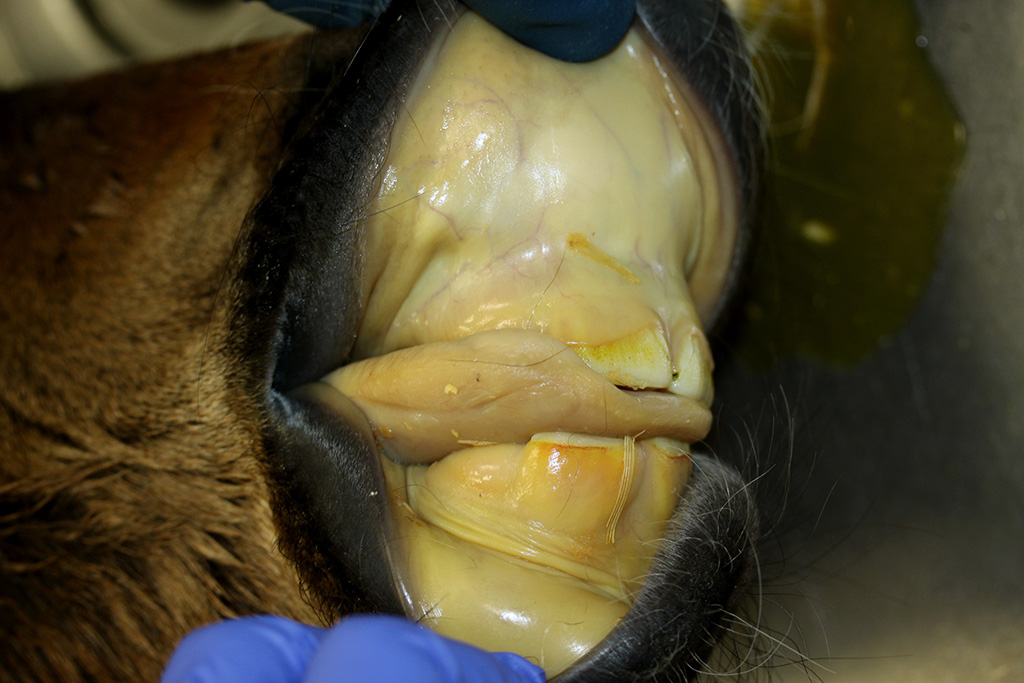
Gross Description:
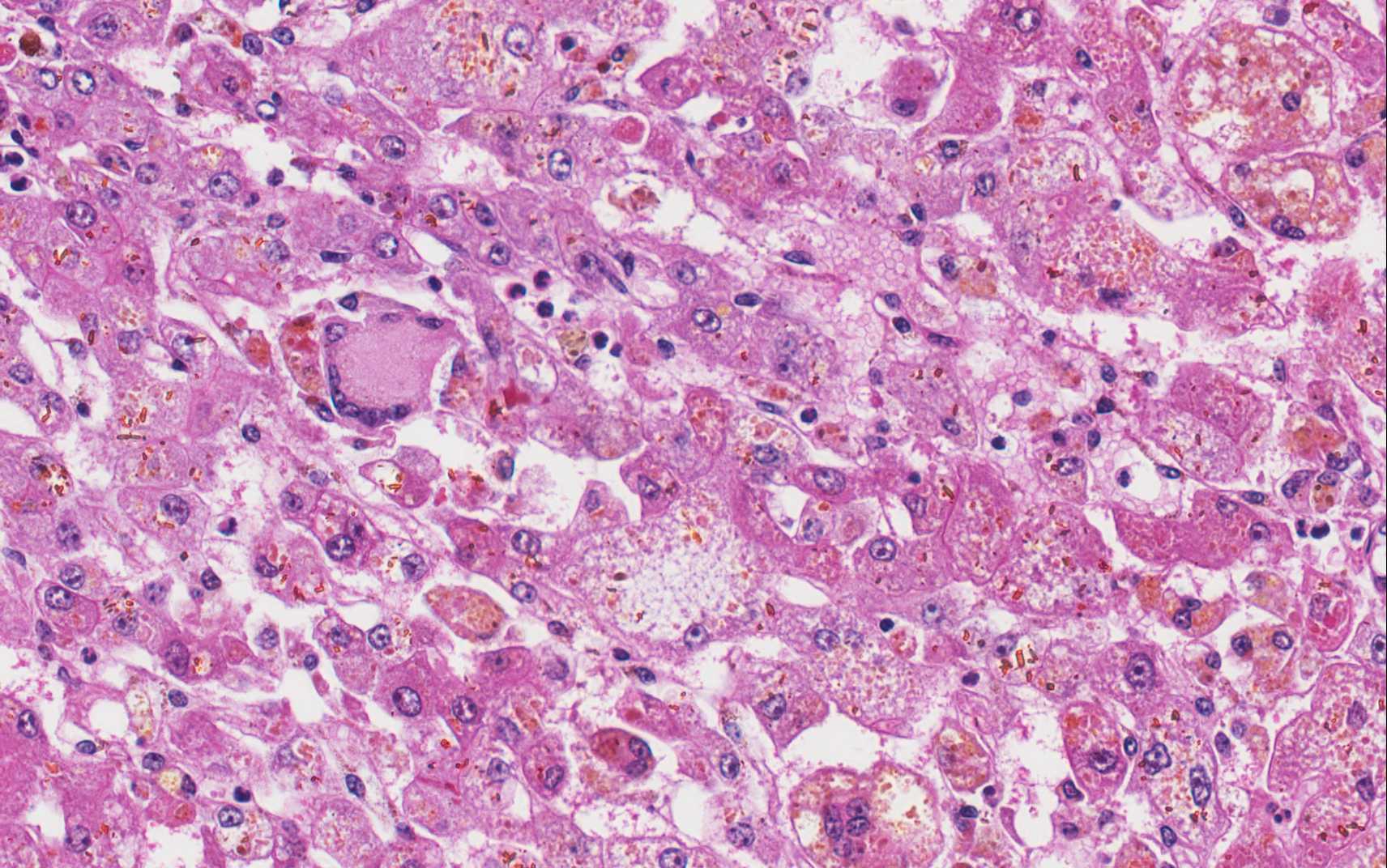
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
PCV=12 (32-53) no Nrbcs
Platelets 470,000/ul (100-350,000)
TP 4.7g/dl (5.3-7.3)
Total bilirubin 14.5 mg/dl (0-1.9)
GGT 124U/L (0-53)
Alkaline phosphatase 553U/L (128-512)
ALT 850U/L (134-643)
Saline agglutination test positive
Mare serum Anti-Qab positive
Condition:
Contributor Comment:
When reviewing the case with the resident, the pathologist commented that the giant cells were classically seen with NI; however, the resident noted that this lesion is not mentioned in textbooks as a lesion associated with NI. Young horse hepatocytes often form syncytia, and giant cell hepatopathies/ hepatidides are described; especially associated with leptospirosis,(13,20) but idiopathic hepatocyte syncytia have been described in 5-7 month-old, equine abortuses(3) and have been seen by this contributor in leptospira PCR-negative cases. In human infants with jaundice, it was described as giant cell hepatitis(17) or syncytial giant cell hepatitis.*(15) Initially, some considered it an expression of viral hepatitis, particularly serum hepatitis, in infancy. Some feel the lesion reflects a specific insult such as blood group incompatibility between mother and infant. It has been considered a nonspecific response of liver regeneration to any injury.(10,19) Still others consider it a congenital defect in the formation of bile canaliculi or another genetically transmitted abnormality.(5,14) Popper and Schaffner(14) concluded hepatocyte giant cell formation was a feature of cholestasis in infancy, and that it may be associated with some inflammatory changes. Syncytial cell hepatitis has been reported in humans and is associated with bacterial sepsis (especially toxoplasmosis, syphilis listeriosis, tuberculosis), viral diseases (cytomegalovirus, herpes simplex, varicella, echovirus, parvovirus B19, enterovirus, rubella, human herpesvirus-6, human immunodeficiency virus, hepatitis types A, B and C, Marburg virus and paramyxovirus), liver transplantation and death from severe liver failure.(8,16) However, in the neonatal period it is considered a non-specific reaction of immature hepatocytes to various forms of aggression,10 and the syncytia stain with nuclear proliferation and growth factors.(7) Just as in autoimmune hemolytic anemia in early childhood, syncytial cell hepatopathy has been reported in cases of equine NI.(2) It was noted in a case of NI presented in the WSC of 5-14-86. How does one explain the lack of the lesion description? Foal livers in fatal cases of NI are busy, and the syncytial cells, while typical, may be down-played in face of the catastrophic clinical and autopsy findings. Because our cases are often referral cases and are subacute to chronic, it may also be that syncytia are just more frequent in long-standing, fatal cases. Syncytia are characteristic. Foals seem prone to respond this way. Although neonatal infections may complicate clinical NI, infectious agents are not always documentable. We should remember that only recently a viral agent of equine Theilers disease has been identified,(4) which may play a role (though I doubt it).
Kernicterus is always a concern in cases of prolonged unconjugated hyperbilirubinemia in neonates. This foal had no macroscopic lesions of kernicterus, the cortex did not fluoresce and no neuronal lesions were noted in the cerebral cortex, Purkinje cells or hippocampus, where it has been described in foals with NI.(12) Its of interest that syncytia were not reported in any of their autopsy cases, even their chronic cases. There is a case report of NI in a three-day-old foal with kernicterus and neuronal necrosis represented macroscopically by yellow-discolored nuclei.(6)
For completeness, it is interesting that this colt had a persistent neutrophilia and developed a thrombocytosis. Alloimmune, neonatal neutropenia has been described in a foal with Ka antibodies in a recent report of NI.(21) The most complete study of antierythrocyte antibodies in mare serum and colostrum was done in thoroughbred and standardbred mares and Qa and Aa seemed to be the most common alloantibodies found at large;(1) however, these alloantibodies may not be the most common seen in clinical cases of NI.(2, 9) Alloantibodies to Qa antigens were found in the mare of our case. The colt of our study did not show a regenerative erythroid response. Over the 2-week hospitalization, no nucleated erythrocytes were noted and the bone marrow had erythrocytic hypoplasia. We wondered if this may have reflected a specific destruction of precursors of the erythroid series.
JPC Diagnosis:
Conference Comment:
Lipofuscin is an intracellular pigment thought to accumulate as a result of aging and cellular wear and tear, including processing of senescent cellular organelles and other material. It accumulates within lysosomes most significantly in post mitotic or slowly dividing cells as an end product of autophagy and is often seen as a perinuclear golden brown pigment. It looks very similar histologically to ceroid, which is more commonly associated with pathologic conditions such as severe malnutrition and vitamin E deficiency or can be seen in certain inherited conditions such as neuronal ceroid lipofuscinosis (see WSC 2015-16, conference 10, case 1). Ceroid is known to accumulate in both Kupffer cells and hepatocytes as well as other tissues and can be intracellular or extracellular. Lipofuscin is thought to accumulate slowly over the life of the animal, whereas ceroid usually accumulates rapidly depending on the associated condition. Histochemical tech-niques which can aid in identifying both ceroid and lipofuscin include Sudan black, oil-red-O, PAS and Ziehl-Neelsen stains. Although differences in content of the two pigments have been demonstrated by special histochemical techniques, it is not possible to differentiate the two pigments in standard H&E stained sections.(11)
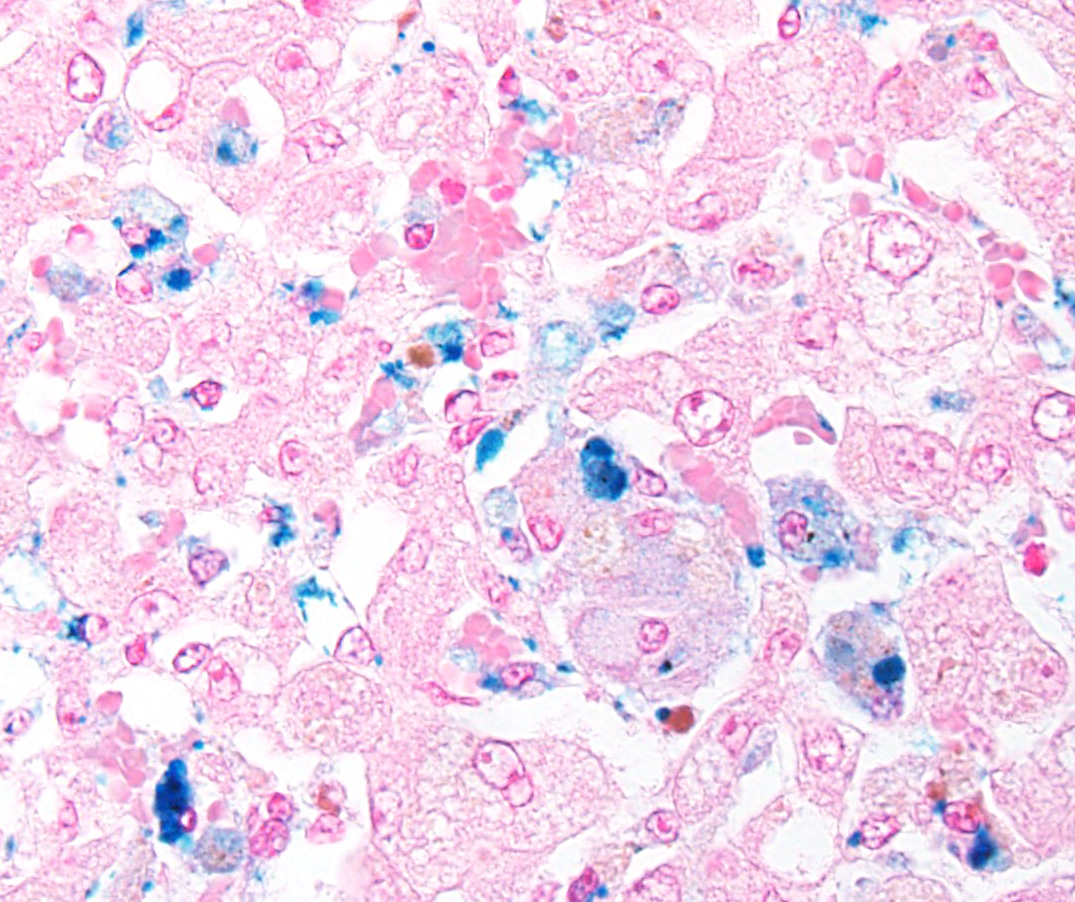
The primary histologic features in this case include hepatocyte degeneration and atrophy, with formation of syncytial cells. Extensive pigmentation of hepatocytes and Kupffer cells was present throughout the section prompting extensive discussion. The brown granular to globular intracellular pigment within hepatocytes was interpreted as lipofuscin and within Kupffer cells as hemosiderin. An iron stain confirmed the presence of moderate amounts of hemo-siderin within Kupffer cells and occasionally within hepatocytes. Viewing under fluor-escence confirmed the presence of small amounts of autoflourescent pigment within hepatocytes consistent with lipofuscin. Abundant birefringent, yellow-brown spiculated material was ultimately identified as acid hematin. Numerous dilated bile canal-iculi were also noted, indicating cholestatic disease. A Halls stain for bile confirmed this finding.
We thank the contributor for providing clinical pathology data with the submission, which greatly adds to the teaching value of the case. One of the best indicators of regenerative anemia is reticulocyte count, but horses do not release reticulocytes. The absence of nucleated red blood cells may suggest a nonregenerative anemia; however, the best way to determine regenerative status would be a bone marrow sample. In this case, a bone marrow aspirate revealed erythrocytic hypoplasia, as indicated above in the contributors comment. Conference participants briefly discussed bilirubin metabolism and the elevated total bilirubin in this case was interpreted as both pre-hepatic and hepatic, which fits with both the pathogenesis and histologic findings.
References:
1. Bailey E. Prevalence of anti-red blood cell antibodies in the serum and colostrum of mares and its relationship to neonatal isoerythrolysis. Am J Vet Res. 1982; 43:1917-21.
2. Boyle A, Magdesian KC, Ruby RE. Neonatal isoerythrolysis in horse foals and a mule foal: 18 cases (1988-2003). J Am Vet Med Assoc. 2005; 227: 1276-1283.
3. Car BD, Anderson WI. Giant cell hepatopathy in three aborted midterm equine fetuses. Vet Pathol. 1988; 25:389-91.
4. Chandriani S, et al. (2013) Identification of a previously undescribed divergent virus from the Flaviviridae family in an outbreak of equine serum hepatitis. ProcNatl Acad Sci U S A. 2013 Apr 9; 110(15):E1407-15. doi: 10.1073/pnas.1219217110.
5. Clayton PT, Disorders of bile acid synthesis. J Inherit Metab Dis. 2011; 34:593-604.
6. David JB, Byers TD, Braniecki A, Chaffin MK, Storts RW. Kernicterus in a foal with neonatal isoerythrolysis. Comp Cont Educ Prac Veterinarian. 1988; 20:517-20.
7. Fang JWS, Gonz+â-ílez-Peralta RP, Chong SKF, Lau GM. Hepatic expression of cell proliferation markers and growth factors in giant cell hepatitis: Implications for the pathogenetic mechanisms involved. JPGN. 2010; 52:65-72.
8. Hicks J, Barrish J, Zhu SH. Neonatal syncytial giant cell hepatitis with paramyxoviral-like inclusions. Ultrastructural Pathol. 2001; 25:65-71.
9. MacLeay JM. Neonatal isoerythrolysis involving Qc and Db antigens in a foal. J Am Vet Med Assoc. 2001; 219:79-81.
10. Maggiore G, Sciveres M, Fabre M, Gori L, et al. Giant cell hepatitis with auto-immune hemolytic anemia in early childhood: Long-term outcome in 16 children. J Pediat. 2012; 159:127-132.
11. Myers RK, McGavin MD, Zachary JF. Cellular adaptions, injury and death: Morphologic, biochemical and genetic bases. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby Elsevier; 2012:43-44.
12. Polkes AC, Giguere S, Lester GD, Bain FT. Factors associated with outcome in foals with neonatal isoerythrolysis (72 Cases, 1988 2003). J Vet Intern Med. 2008; 22:1216-1222.
13. Poonacha KB, Smith BJ, Donahue JM, Tramontin RR. Leptospiral abortion in horses in central Kentucky. Proc 36th Ann Convention Am Asso Eq Practitioners. 1991; 36:397-402.
14. Popper H, Schaffner F. Pathophysiology of cholestasis. Hum Pathol. 1970; 1:1-24.
15. Portenza L, Luppi M, Barozzi P, Rossi G. HHV-6 in syncytial giant cell hepatitis. NE J Med. 2008; 359:593-602.
16. Raj S, Stephen T, Debski RF. Giant cell hepatitis with autoimmune hemolytic anemia: A case report and review of pediatric literature. Clin Pediatr. 2011; 50:357-9.
17. Shaffner F, Popper H. Morphologic studies in neonatal cholestasis with emphasis on giant cells. Ann NY Acad Sci. 1963; 111:358-374.
18. Snyder PW. Diseases of immunity. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby Elsevier; 2012:263-264.
19. Torbenson M, Hart J, Westerhoff M, Azzam RK. Neonatal giant cell hepatitis: Histological and etiological findings. Am J Surg Pathol. 2010; 34:1498-1503.
20. Wilke IW, Prescott JF, Hazlett MJ, Maxie MG, van Dreumel AA. Giant cell hepapatitis in four aborted foals: A possible leptospiral infection. Can Vet J. 1988; 29:1003-4.
21. Wong DM, Alcott CJ, Clark SK, Jones DE. Alloimmune neonatal neutropenia and neonatal isoerythrolysis in a Thoroughbred colt. J Vet Diag Invest. 2013; 24: 219-226.