Wednesday Slide Conference 2023-2024, Conference 5 Case 4
Signalment:
12-day-old, Charolais bull, bovine (Bos taurus)
History:
The calf was unwell for 1 week and unable to suck with no response to antibiotic treatment. Only one calf was affected.
Gross Pathology:
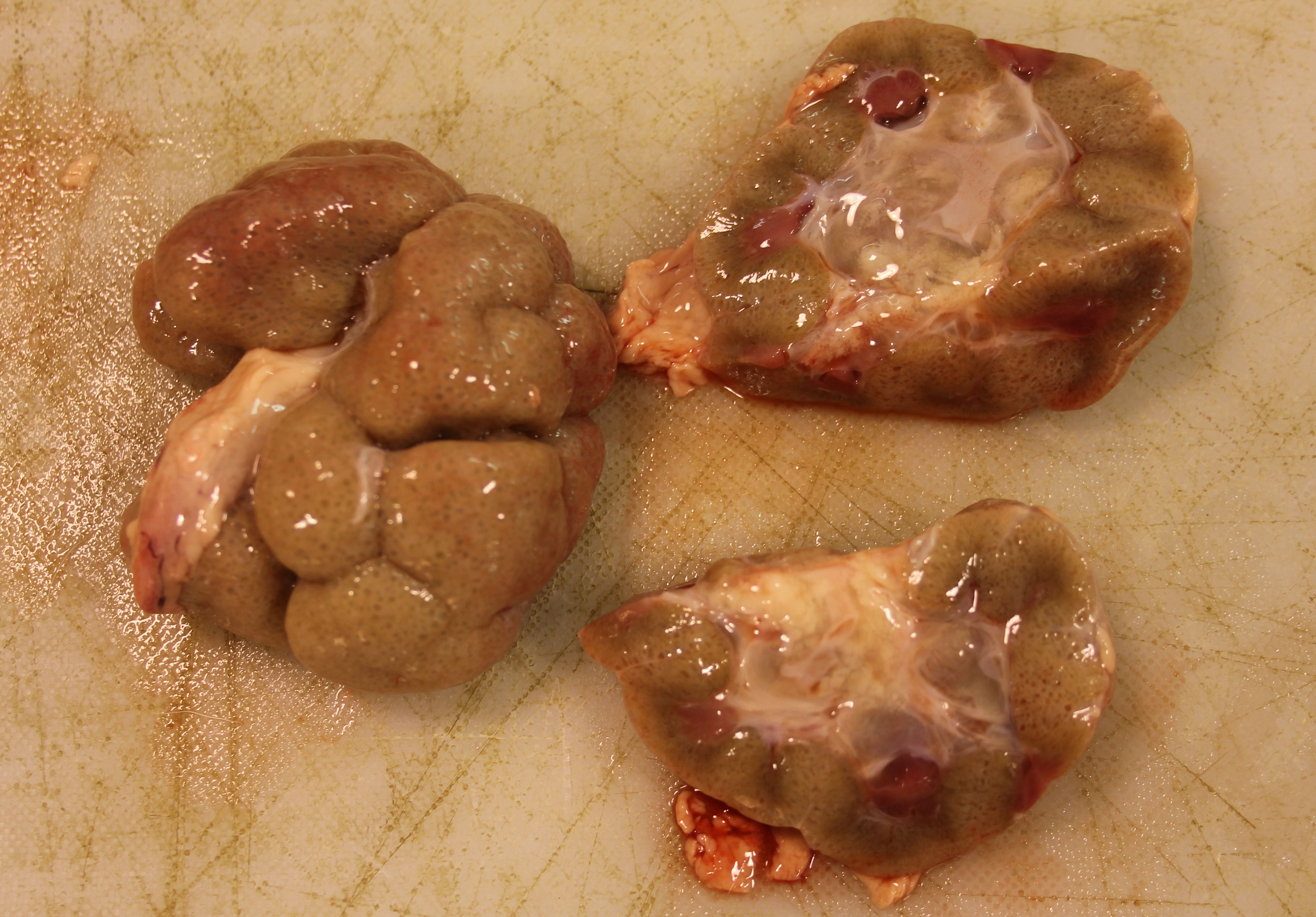
The patient weighed 50 kg and was in age-appropriate body condition. Bilaterally the kidneys were tan-grey with an uneven subcapsular surface featuring multifocal pinpoint indentations. The calyces were oedematous.
Laboratory Results:
Urinalysis: Glucose = 14mml/l; Specific Gravity = 1.030; Protein = 2g/l; pH = 6; moderate number of nitrates; no leucocytes, urobilinogen, or RBCs.
Liver selenium: 71.50 umol/kg (normal range =5-20 umol/kg).
Zinc Sulphate Turbidity: Adequate colostral immunity.
Microscopic Description:
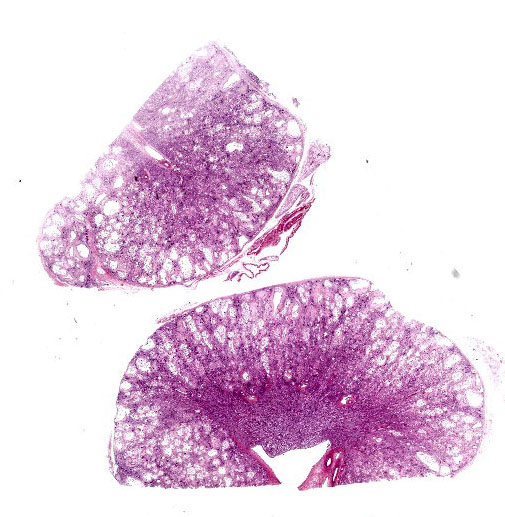
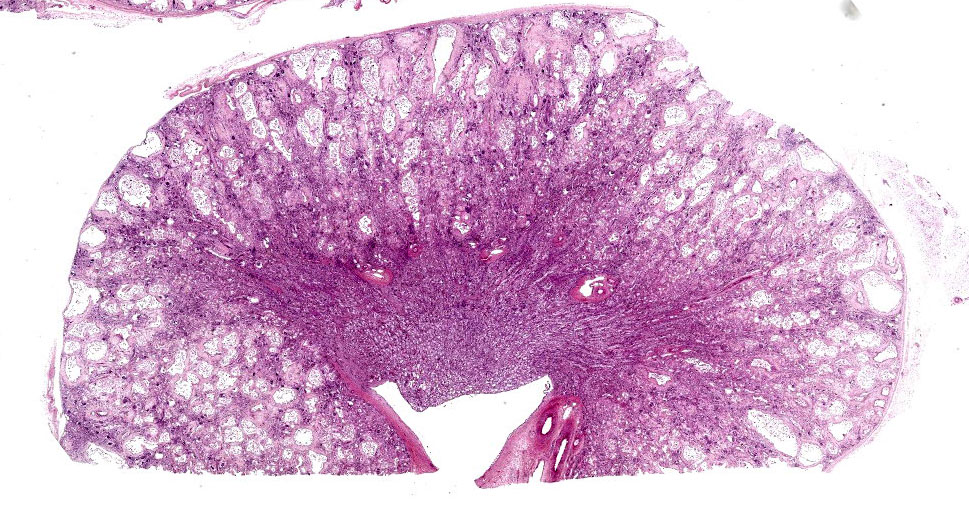
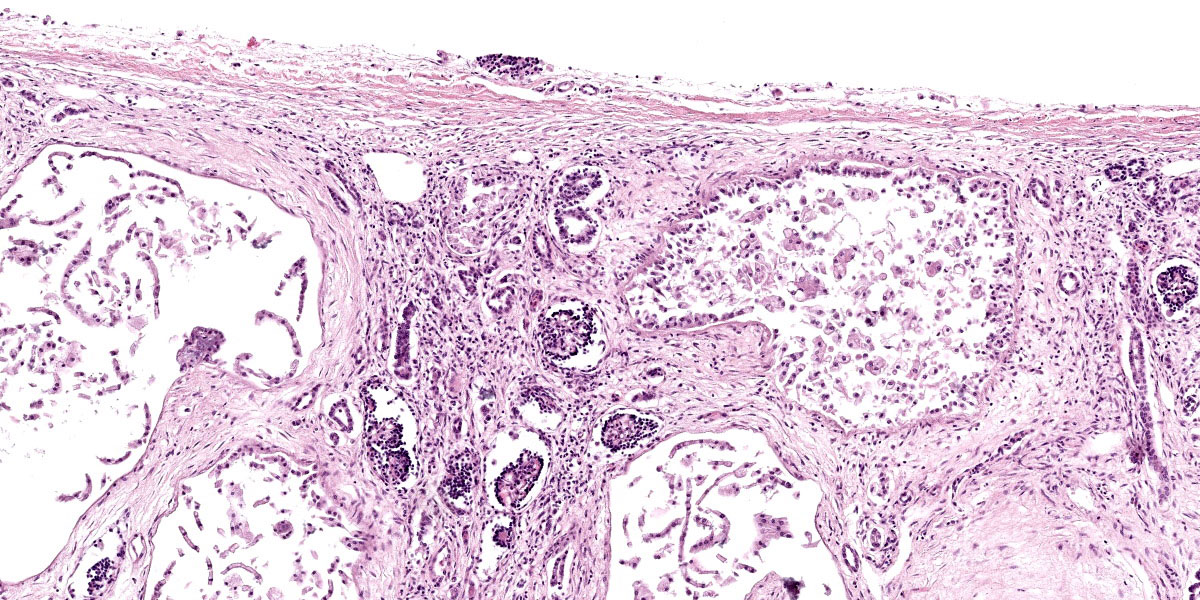
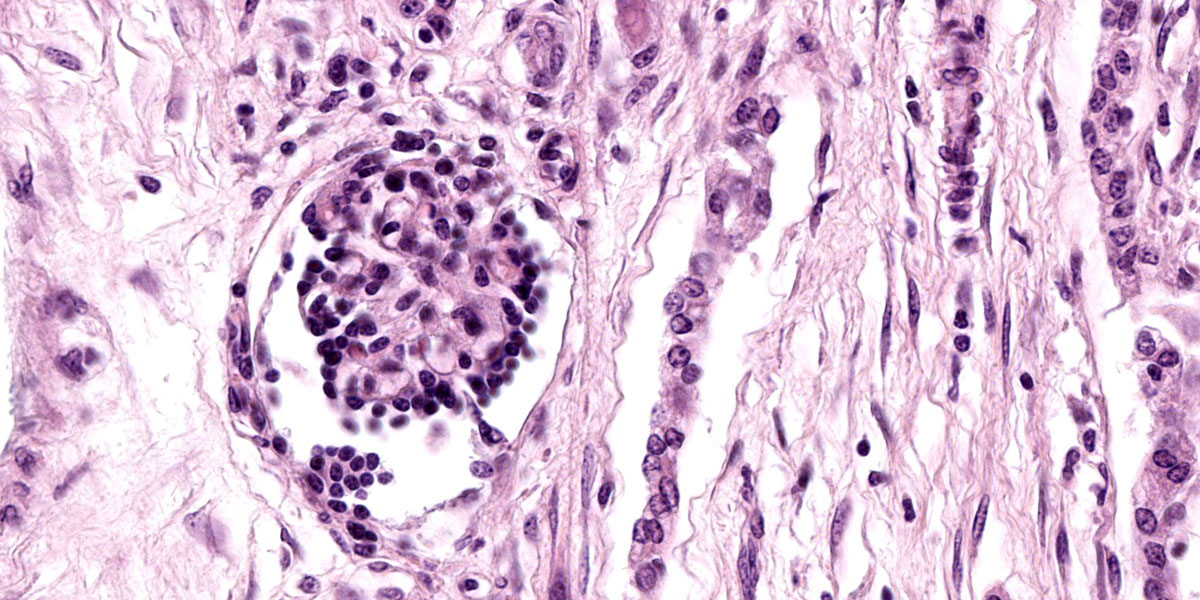
Kidney: The cortex is diffusely and markedly disrupted by large numbers of cystic spaces up to 120 um in diameter lined by a monolayer of cuboidal epithelium that is multifocally exfoliated and floating freely within these cystic lumens (autolysis). These cysts are separated by a large amount of fibro-myxoid, oedematous stroma that is composed of collagen bundles and plump fibroblasts. Multifocally, and predominantly within the cortex, are variably sized areas that exhibit pale eosinophilic, glassy, loose stroma with scattered stellate cells (primitive mesenchyme). Multifocally the glomeruli are small and mildly to moderately hypercellular with occasionally mildly thickened Bowman’s capsules. Multifocally some glomeruli are irregularly lobulated. Multifocally the cortical tubules exhibit a small amount of luminal radiating achromatic crystalline material that is birefringent under polarized light (calcium oxalate crystals). Multifocally some of the cortical tubular epithelium exhibits a small amount of yellow, globular intracytoplasmic pigment. Multifocally medullary tubules exhibit dark basophilic epithelium that occasionally undergoes ‘piling up.’ Multifocally lumens within the medulla exhibit a small amount of eosinophilic, homogenous to glassy material (protein casts). Very rarely some of the tubules at the cortico-medullary junction exhibit ciliated epithelium (persistent mesonephric ducts). Multifocally interstitial blood vessels exhibit mildly thickened tunica media. Multifocally the stroma is infiltrated by small to moderate numbers of lymphocytes and plasma-cells (interstitial nephritis).
Contributor’s Morphologic Diagnoses:
- Kidney: Asynchronous maturation, interstitial fibrosis, with fetal glomeruli, immature tubules, primitive mesenchyme, persistent metanephric ducts, consistent with renal dysplasia.
- Kidney: Lymphoplasmacytic nephritis, chronic, mild.
Contributor’s Comment:
Renal dysplasia is defined as disorganised development of renal parenchyma due to anomalous differentiation and has been described in numerous species including humans, sheep, pigs, cats, horses and, most frequently, dogs.2,11 The cause(s) of renal dysplasia are not well understood but are likely to be multifactorial and in some cases are considered congenital.2 Proposed causes include genetic mechanisms, as well as in-utero infections and deficiencies affecting fetal development. Renal dysplasia has been identified in multiple dog breeds including the Golden Retriever, Beagle, Lhasa Apso, Great Dane, Dutch Kooiker dog, Samoyed, Alaskan Malamute, Cavalier King Charles Spaniel and Bulldog.1,7,11,13 Renal dysplasia with hepatic fibrosis was described in Norwich terriers with INPP5E splice site variant causing lethal ciliopathy.3 Examples of fetal infections resulting in renal dysplasia include panleukopenia virus in cats, canine herpes virus in dogs and BVDV infection in cattle.10 In pigs, hypovitaminosis A is considered a cause of renal dysplasia.2
The gross presentation of the kidneys of affected animals varies, but affected kidneys are usually small, misshapen, fibrosed, and feature multiple cysts and tortuous ureters. One or both kidneys may be affected in an individual. In some cases, dysplastic kidneys show only minimal gross changes, such as having a slightly irregular contour.2 The histopathological criteria to achieve a diagnosis of renal dysplasia include:
- The presence of anomalous or undeveloped structures;
- The presence of undifferentiated mesenchyme in the cortex or medulla;
- Groups of immature glomeruli in non-neonatal animals;
- Lack of glomeruli or tubules in some lobes of the kidneys;
- Collecting tubules with blind ends within cortical connective tissue;
- Atypical tubular epithelium;
- The presence of primitive (metanephric) ducts lined by cuboidal or columnar epithelium; and
- Dysontogenic (cartilaginous or osseous) metaplasia, mainly seen in humans.2
Because ureteral anomalies are often present in cases of renal dysplasia, dysplastic kidneys are also highly susceptible to developing pyelonephritis. Various forms of renal dysplasia and nephropathy have been described in ruminants, including: renal dysplasia in Japanese black cattle;12 autosomal dominant cystic renal dysplasia in Suffolk sheep;8 renal dysplasia with hydronephrosis and congenital ureteral strictures of unknown cause in two Holstein-Friesian calves;15 immune mediated mesangiocapillary glomerulonephritis in Finnish Landrace lambs (cause unknown but thought to represent a recessive, inherited defect of the complement system);5 nephropathy in Japanese black cattle caused by a claudin 16 gene deletion mutation;6 and familial glomerulopathy and peripheral neuropathy in Gelbvieh cattle.9
The cause of the renal dysplasia in the case presented here remains unknown. Both calf and dam were negative for BVDV and no abnormalities were found in other tissues. The significance of the very high liver selenium concentration remains unclear.
Contributing Institution:
Veterinary Sciences Centre
School of Veterinary Medicine
University College Dublin
Belfield, Dublin 4, Ireland
http://www.ucd.ie/vetmed/
JPC Diagnosis:
Kidney: Asynchronous maturation with fetal glomeruli, tubular loss, marked interstitial fibrosis, and mild lymphoplasmacytic interstitial nephritis.
JPC Comment:
Renal dysplasia is a broad term that encompasses a range of gross and histologic abnormalities caused by development gone rogue. In mammals, two precursor kidneys are formed prior to the arrival, in the mature animal, of the metanephric kidney. The first is the pronephros, which contains primitive tubules and a pronephric duct, that grows caudally and terminates in the fetal cloaca.4 The tubules of the pronephros degenerate, but the pronephric duct persists as the mesonephric duct. The mesonephric duct, in turn, induces the formation of the mesonephric kidney, which consists of tubules that terminate in vascular proliferations arising from the dorsal aorta on one end and the mesonephric duct on the other. In amphibians and fish, the mesonephros becomes the functional mature kidney. In reptiles, birds, and mammals, the mesonephros degenerates, setting the stage for the arrival of the metanephros.4
The metanephric kidney beings as an evagination from the mesonephric duct, called the ureteric bud.11 The ureteric bud moves cranially and differentiates into the ureter, renal pelvis, and collecting ducts. The ureteric bud grows into a mass of mesenchymal cells known as the metanephrogenic mass or the metanephric blastema. As the ureteric bud successively divides into collecting ducts, these ducts induce nephrogenic differentiation of the metanephric blastema. These proto-nephrons elongate, canalize and induce the formation of immature glomeruli at one end, and connect with the collecting ducts at the other. Nephrons continue to develop from deep to superficial within the kidney and, in some species, continue to form and mature after birth.
The term renal dysplasia implies disorder of the complex interactions between the ureteric bud and the metanephric blastema, resulting in anomalous metanephric differentiation.11 The contributor outlines several canonical histologic criteria, at least one of which must be present for a diagnosis of renal dysplasia. Two of these criteria, the presence of metanephric ducts surrounded by primitive mesenchyme and the formation of dysontogenic tissues, both indicate total failure of the initial interaction between the ureteric bud and the metanephric blastema.11 In contrast, the presence of fetal glomeruli and tubules, the presence of persistent mesenchyme, and the presence of anomalous structures indicate that the induction of the metanephric blastema was initiated but failed to undergo complete differentiation.11
As the contributor notes, ureteral anomalies are common in cases of renal dysplasia. This is unsurprising given the close developmental relationship between the kidney and the collecting system. Among the reported ureteral anomalies associated with renal dysplasia are ectopic ureters, ureteral obstruction with accompanying hydronephrosis, hydroureter, and congenital urothelial cell hyperplasia.10,14,15
Conference discussion focused on the difficulty of definitively identifying structures, such as persistent metanephric ducts or primitive mesenchyme, amid the histologic noise present in a kidney as dysplastic as the section examined in conference. While fetal glomeruli are more obvious, their presence can be a normal finding in the neonates of some species, such as dogs, in which renal development continues through the first few weeks of life.
Discussion also focused on the term “renal dysplasia,” which has fallen somewhat out of favor due to its relatively elastic meaning which has become somewhat overstretched over time due to inconsistent use. The currently proposed term, “renal maldevelopment,” is meant to describe the disorganized development of renal parenchyma exemplified by this case. The term “renal dysplasia,” while well-ensconced in the veterinary literature, should be thought of as an umbrella term, covering a heterogenous group of renal developmental anomalies.
References:
- Bruder MC, Shoieb AM, Shirai N, Boucher GG, Brodie TA. Renal dysplasia in Beagle dogs: four cases. Toxicol Pathol. 2010;38(7):1051-1057.
- Cianciolo RE, Mohr FC. Urinary system. In: Maxie MG, ed. Jubb, Kennedy,and Palmer’s Pathology of Domestic Animals. Vol. 2. 6th ed. Philadelphia, PA: Elsevier; 2016:393.
- Dillard KJ, Hytönen MK, Fischer D, et al. A splice site variant in INPP5E causes diffuse cystic renal dysplasia and hepatic fibrosis in dogs. PLoS One. 2018;13(9):e0204073.
- Fletcher TF, Weber AF. Veterinary developmental anatomy (veterinary embryology). University of Minnesota College of Veterinary Medicine. 2013. Accessed September 20, 2023. http://vanat.cvm. umn.edu/vanatpdf/EmbryoLectNotes.pdf.
- Frelier PF, Armstrong DL, Pritchard. Ovine mesangiocapillary glomerulonephritis type I and crescent formation. Pathol. 1990;27(1):26-34.
- Hirayama H, Kegeyama S, Moriyasu S, et al. Genetic diagnosis of claudin 16 deficiency and sex determination in bovine preimplantation embryos. Reprod. Dev. 2004; 50:613-618.
- Kerlin R., Van Winkle TJ. Renal dysplasia in Golden Retrievers. Pathol. 1992; 32:327-329.
- O‘Toole D, Jeffrey M, Jones T, Morgan G, Green R. Pathology of renal dysplasia and bladder aplasia-hypoplasia in a flock of sheep. Vet. Diagn. Invest. 1993; 5:591-602.
- Panciera RJ, Washburn KE, Streeter RN, Kirkpatrick JG. A familial peripheral neuropathy and glomerulopathy in Gelbvieh calves. Pathol. 2003;40 (1):63-70.
- Percy DH, Carmichael LE, Albert DM, King JM, Jonas AM. Lesions in puppies surviving infection with canine herpesvirus. Vet Pathol. 1971;8(1):37-53.
- Picut CA, Lewis RM. Microscopic features of canine renal dysplasia. Vet Pathol. 1987;24(2):156-163.
- Sugiyama A, Ozaki K, Miyazaki, Tanabe Y, Takeuchi T, Narama I. Renal dysplasia unrelated to claudin-16 deficiency in Japanese Black cattle. J Comp Pathol. 2007;137(1):71-77.
- Whiteley MH, Bell JS, Rothman DA. Novel allelic variants in the canine cyclooxgenase-2 (Cox-2) promoter are associated with renal dysplasia in dogs. PLoS One. 2011;8;6(2):e16684.
- Yoon HY, Mann FA, Punke JP, Jeong SW. Bilateral ureteral ectopia with renal dysplasia and urolithiasis in a dog. J Am Anim Hosp Assoc. 2010;46(3):209-214.
- Yoshida K, Takezawa S, Itoh M, Takahashi E, Inokuma H, Watanabe K, et al. Renal dysplasia with hydronephrosis and congenital ureteral stricture in two Holstein-Friesian calves. J Comp Pathol. 2022;193:20-24.