Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 16
January 24th, 2018
CASE I: F16-0079.3 (JPC 4101317).
Signalment: 5-year-old, male, Green tree python, Morelia viridis, reptile.
History: The animal showed acute respiratory distress and discharge of mucus from the oral and nasal cavity. Two days after the onset of symptoms the animal was found dead.
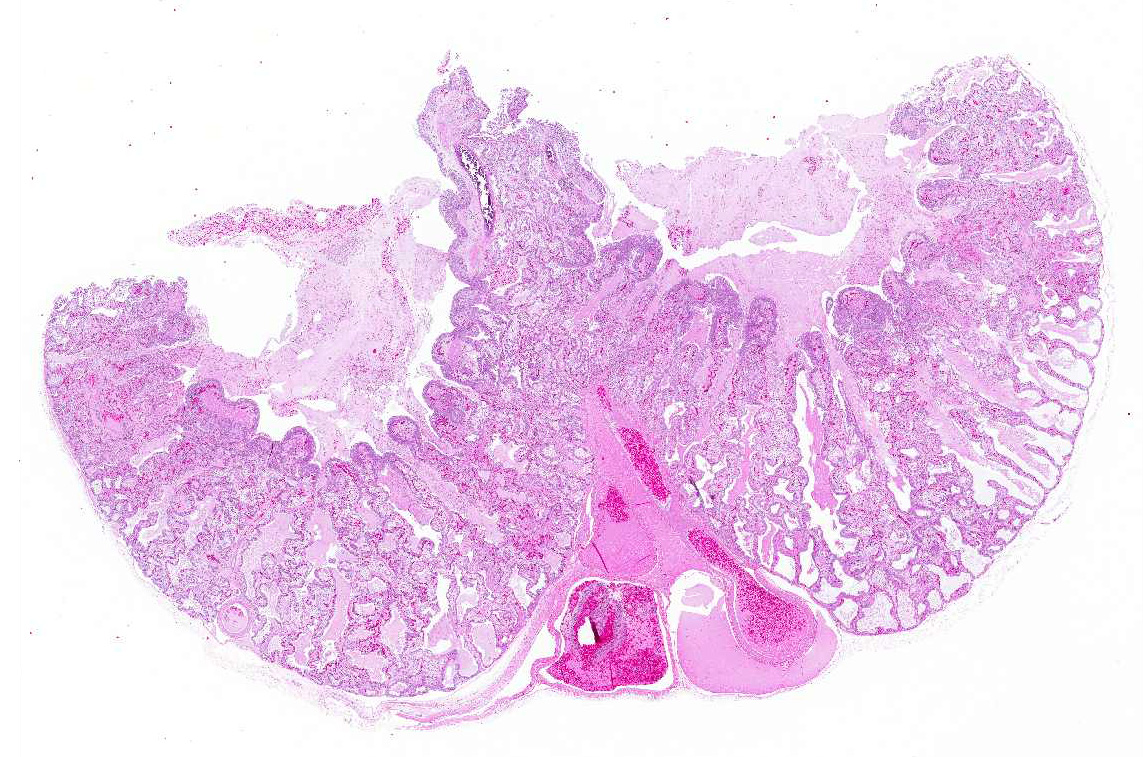
Gross Pathology: A marked thickening of the lung parenchyma and a severe accumulation of clear mucus in the upper and distal airways (larynx, trachea, lung, air sacs) were noted at gross examination. The animal was in moderate body condition and the carcass was slightly dehydrated. All other internal organs showed no macroscopic changes.
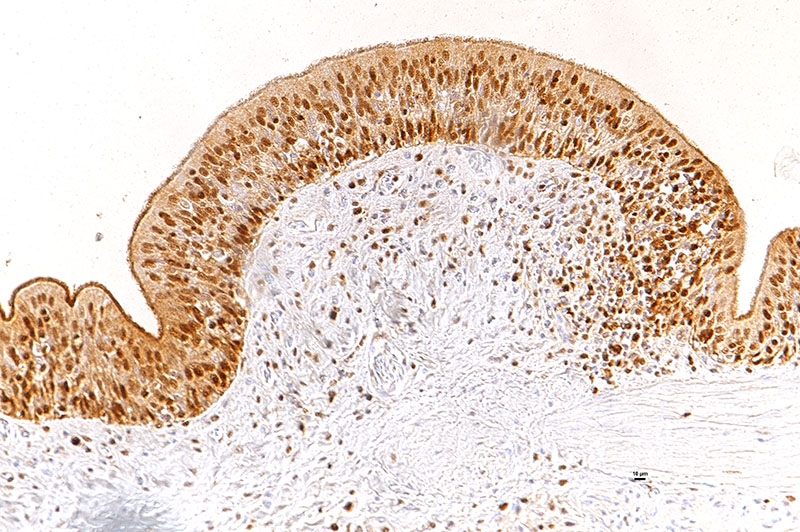
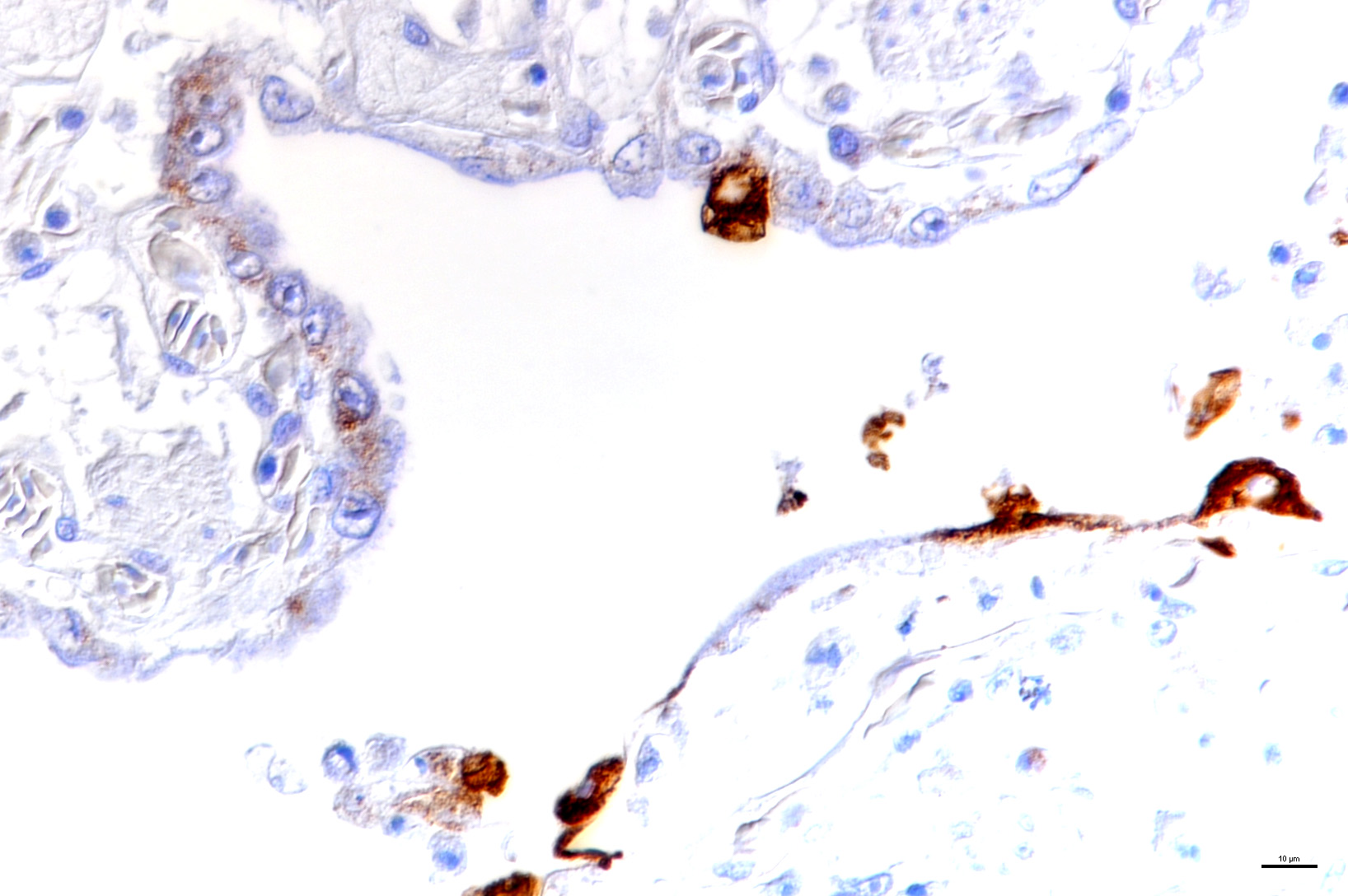
Laboratory results: The presence of nidoviral RNA (Morelia viridis Nidovirus, “MVNV”) was confirmed through RT-PCR of the affected lung tissue and intracellular nidoviral protein was detected via immunohistochemistry in respiratory and faveolar epithelial cells of the lungs. Infected epithelial cells were also found in the trachea and the nasal cavity. A markedly increased proliferative activity was noted in all epithelial cell layers of the diseased lung via a PCNA marker. Additionally, the immunohistochemical examination for the detection of active-caspase 3 revealed an increased number of apoptotic epithelial cells in the lung of the infected animals.
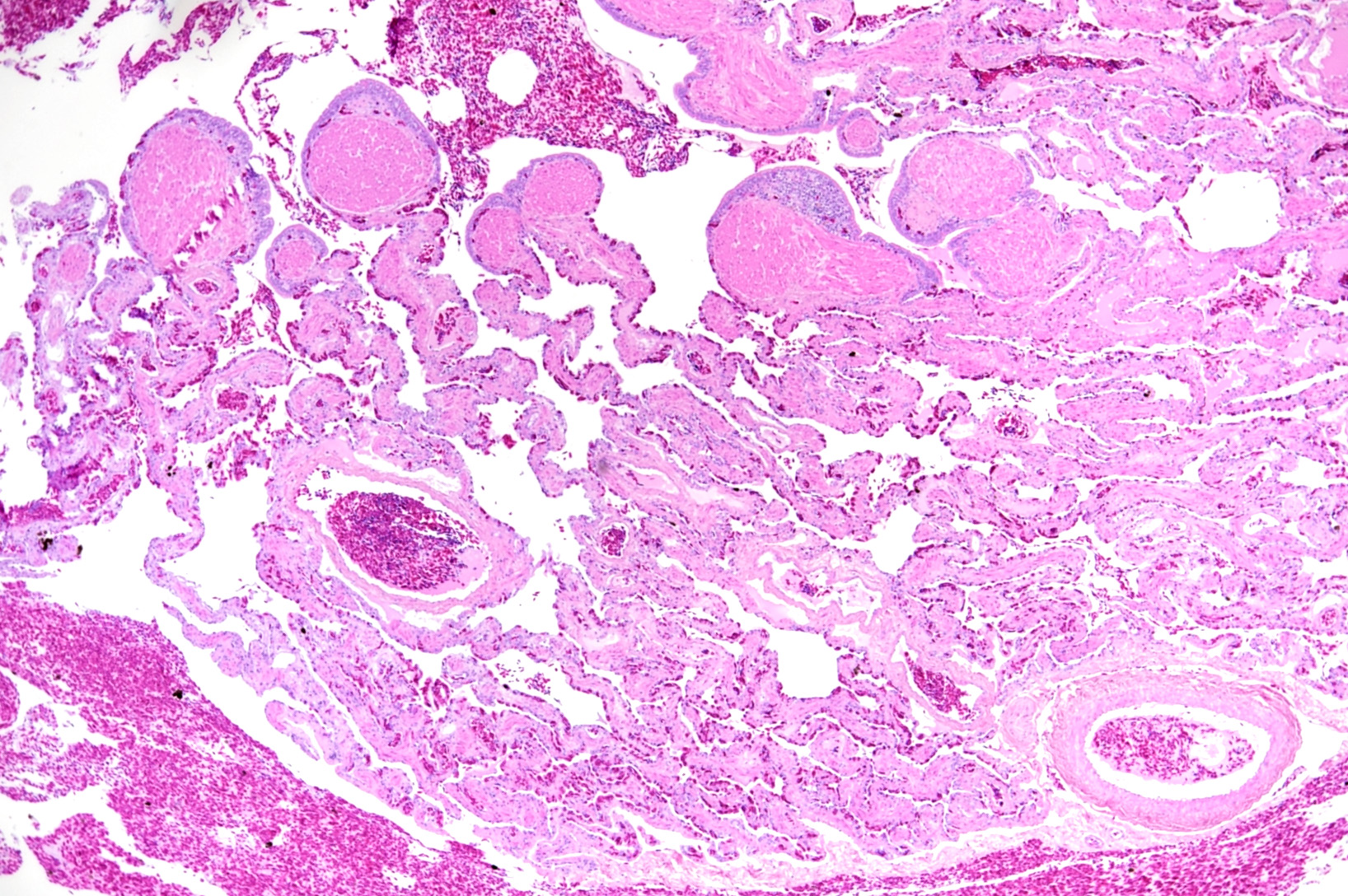
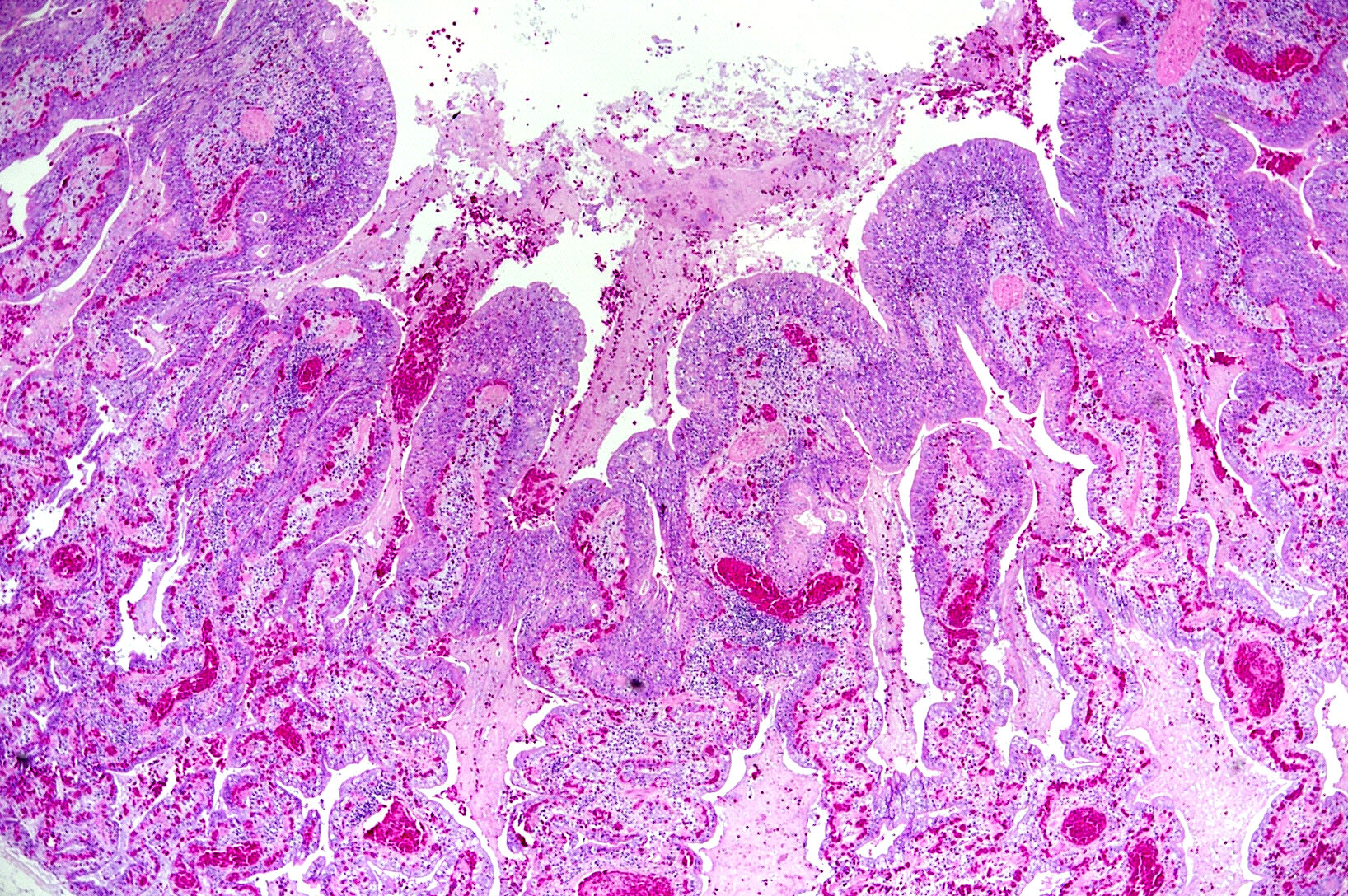
Microscopic Description: The two histologic hallmarks are the epithelial thickening in the entire lung and excess mucus in the lumen of lungs and airway. The multilayered respiratory epithelium covering the smooth muscle at the luminal end of the trabeculae displays increased cellularity with an increase in cell layers and irregular cell arrangement (hyperplasia). In the faveolar space, the epithelium is characterized by patchy to diffuse proliferations of cuboidal to columnar cells carrying short microvilli (type II pneumocytes) and rare flat cells with short cytoplasmic projections covering the capillaries (type I pneumocytes). The inflammatory component is represented by moderate, multifocal to diffuse interstitial infiltration of lymphocytes, plasma cells and heterophilic granulocytes. Multifocal heterophilic granulocytes and lymphocytes also infiltrate the respiratory epithelium. The lymphoid aggregates (equivalent to the mammalian bronchiolar-associated tissue) are activated. Abundant homogenous eosinophilic fluid (mucus) is filling the faveolar space, admixed with numerous heterophilic granulocytes, erythrocytes and cell debris.
Contributor’s Morphologic Diagnosis:
Lung: Proliferative pneumonia, severe, diffuse with type II pneumocyte hyperplasia and mucus accumulation in the faveolar space.
Contributor’s Comment: The Morelia viridis nidovirus belongs to the subfamily of the Torovirinae (Family Coronaviridae), which have so far mainly been associated with enteric diseases (humans, horses, cattle, swine).4,11,13,16 However, recent studies on toroviruses have shown that they can be both entero- and pneumotropic.14 In the last two years snake nidoviruses have been identified as the causative agent of a severe respiratory disease in ball pythons, green tree pythons3 and Indian pythons.2,8,9,12 Nidoviruses were also identified in the lungs of cattle and wild shingleback lizards with pneumonia, though their direct association with disease has so far not been examined.9,14
In the python lung, nidovirus infection is associated with a distinct proliferative activity and a degree of apoptotic cell death of both type I and type II pneumocytes, leading to an increased epithelial turnover and hyperplasia.3
In the faveolar space of the healthy snake lung, thin cytoplasmic extensions of the type I pneumocytes cover the capillary walls, forming the gas-blood barrier.10 This case nicely demonstrates a marked replacement of type I pneumocytes by type II pneumocytes in the faveolar space, known as an unspecific regenerative response of snakes to infectious agents, similar to response to lung injury in mammals.5 In the healthy reptilian lung, lamellated bodies containing surfactant can be demonstrated through electron microscopy in type II pneumocytes. Following the nidoviral infection, “transformed” type II pneumocytes containing intracytoplasmic serous/mucous granules are noted ultrastructurally in the faveolar space.3 This epithelial cell type has been previously described in various reptile species and is likely to explain the pathognomonic excess in mucus production in nidoviral pneumonia7,10 and indicate an decrease in surfactant production.3 In the present case viral protein could be detected in the epithelium of the entire respiratory tract (nasal cavity, larynx, trachea, lung and air saccs) and indicates a respiratory transmission (droplet) as the most likely infection route. Nidoviruses cause sudden outbreaks with high mortality in breeding collections, symptoms prior to deceasing/death are rare.
JPC Diagnosis: Lung: Pneumonia, bronchointerstitial, necrotizing, diffuse, mild with marked respiratory and type II pneumocyte hyperplasia and faveolar edema, Green tree python (Morelia viridis), reptile.
Conference Comment: Nidoviruses are a diverse group, affecting humans and a growing population of veterinary species including: snakes, wild shingleback lizards, cattle, and nematodes. Nidoviruses that affect animal species are from the Coronaviridae family and Torovirinae subfamily. Specifically, the Torovirus genus affects mammals and the little known Bafinivirus genus affects ray-finned fish. There have been disagreements regarding the classification of reptile nidoviruses which have not been formally classified to date.1 Regardless of the phylogenetic differences, toroviruses all share similar tissue tropism for the gastrointestinal and respiratory tracts.
Snake-associated nidoviruses were first identified in ball pythons (Python regius) and Indian rock pythons (P. molurus) in 20142,12,15 and green tree pythons (Morelia M. viridis) in 2017.3 Gross findings in these snakes include: stomatitis, sinusitis, pharyngitis, tracheitis, esophagitis, and proliferative pneumonia with abundant mucus secretion.
More and more over the past several years, nidovirus infection has been associated with fatal respiratory disease in several species of python, but Koch’s postulates had not definitively proven that it was the cause. In a recent study,6 it was demonstrated through experimental infection of three ball pythons (Python regius) that nidovirus infection results in mucinous chronic inflammation and proliferative interstitial pneumonia. Possible transmission routes were suggested by identifying infectious virus in oral secretions and fecal material. Additionally, it was suggested that choanal and oral swabs were the best sample locations for antemortem diagnosis.
Conference participants thought there was enough necrosis within the submitted sections to include it as a modifier in the morphologic diagnosis. Morphologically, the participants suggested that the material present within the faveolar spaces suggested proteinaceous edema fluid rather than mucus. The location descriptor of bronchopneumonia as used in the JPC morphologic is based on the presence of hyperplastic and inflammatory changes in the respiratory epithelium at the tips of the faveolar septa as well as the type II pneumoncyte hyperplasia deep in the faveolae.
Contributing Institution:
Institute if Veterinary Pathology
Vetsuisse-Faculty (University of Zurich)
Winterthurerstrasse 268, CH-8057 Zurich
Fax number +41 44 635 89 34
www.vetpathology.uzh.ch
References:
- Adams MJ, Lefkowitz EJ, King AMQ, et al. Changes to taxonomy and the international code of virus classification and nomenclature ratified by the international committee on taxonomy of viruses. Virol. 2017;162:2505-2538.
- Bodewes R, Lempp C, Schurch AC, et al. Novel divergent nidovirus in a python with pneumonia. The Journal of General Virology. 2014;95(Pt 11):2480-2485.
- Dervas E, Hepojoki J, Laimbacher A, Romero-Palomo F, Jelinek C, Keller S, Smura T, Hepojoki S, Kipar A, Hetzel U. Nidovirus-associated proliferative pneumonia in the green tree python (Morelia viridis). (under review in Journal of Virology, 2017).
- Draker R, Roper RL, Petric M, Tellier R. The complete sequence of the bovine torovirus genome. Virus Research. 2006;115(1):56-68.
- Fehrenbach H, Kasper M, Tschernig T, et al. Keratinocyte growth factor-induced hyperplasia of rat alveolar type II cells in vivo is resolved by differentiation into type I cells and by apoptosis. The European Respiratory Journal. 1999;14(3):534-544.
- Hoon-Hanks LJ, Layton ML, Ossiboff RJ, et al. Respiratory disease in ball pythons (Python regius) experimentally infected with ball python nidovirus. 2018 [Epub ahead of print]. doi: 10.1016/j.virol.2017.12.008.
- Jacobson ER, Adams HP, Geisbert TW, Tucker SJ, Hall BJ, Homer BL. Pulmonary lesions in experimental ophidian paramyxovirus pneumonia of Aruba Island rattlesnakes, Crotalus unicolor. Veterinary Pathology. 1997;34(5):450-459.
- Marschang RE, Kolesnik E. Nachweis von nidoviren bei lebenden pythons und boas. Tierarztliche Praxis. Ausgabe K, Kleintiere/Heimtiere. 2017;45(1):22-26.
- O'Dea MA, Jackson B, Jackson C, Xavier P, Warren K. Discovery and partial genomic characterisation of a novel nidovirus associated with respiratory disease in wild shingleback lizards (Tiliqua rugosa). PloS one. 2016;11(11):e0165209.
- Pastor García LM, Murcia Ud, Publicaciones Sd. Histology, ultrastructure and immunohistochemistry of the respiratory organs in non-mammalian vertebrates. [Murcia]: Secretariado de Publicaciones de la Univesidad de Murcia [etc.]; 1995.
- Steele AD, Bos P, Alexander JJ. Clinical features of acute infantile gastroenteritis associated with human rotavirus subgroups I and II. Journal of Clinical Microbiology. 1988;26(12):2647-2649.
- Stenglein MD, Jacobson ER, Wozniak EJ, et al. Ball python nidovirus: A candidate etiologic agent for severe respiratory disease in Python regius. mBio. 2014;5(5):e01484-14.
- Sun H, Lan D, Lu L, Chen M, Wang C, Hua X. Molecular characterization and phylogenetic analysis of the genome of porcine torovirus. Archives of Virology. 2014;159(4):773-778. http://dx.doi.org/10.1007/s00705-013-1861-x.
- Tokarz R, Sameroff S, Hesse RA, et al. Discovery of a novel nidovirus in cattle with respiratory disease. The Journal of General Virology. 2015;96(8):2188-2193.
- Uccellini L, Ossiboff RJ, Matos REC de, et al. Identification of a novel nidovirus in an outbreak of fatal respiratory disease in ball pythons (Python regius). Virology Journal. 2014;11:144.
- Weiss M, Steck F, Horzinek MC. Purification and partial characterization of a new enveloped RNA virus (Berne virus). Journal of General Virology. 1983;64(9):1849-1858.