Signalment:
12.7 yr female Indian rhesus monkey (
Macaca mulatta)This animal was inoculated with SIVB670 (clone12) six years prior to necropsy. It had a long history starting 2 years after inoculation of hair plucking that escalated to self-trauma. Epistaxis was noted from time to time. It was sacrificed due to new self-trauma, depression, and anorexia.
Gross Description:
The surface of the left parietal region of the brain had a 1x2 cm darkened soft depression that extended to the subcortical white matter. Self-trauma to the foot and vegetative valvular endocarditis were noted.
Histopathologic Description:
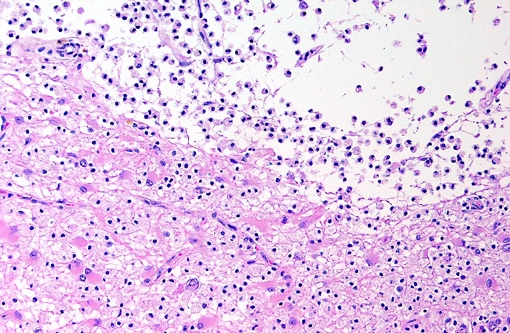
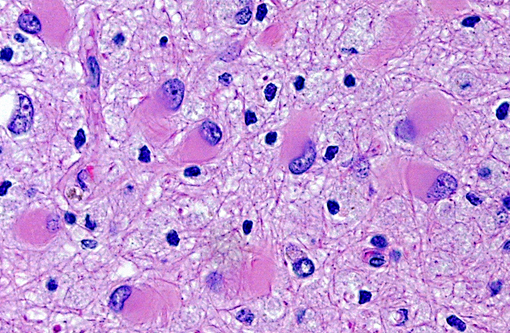
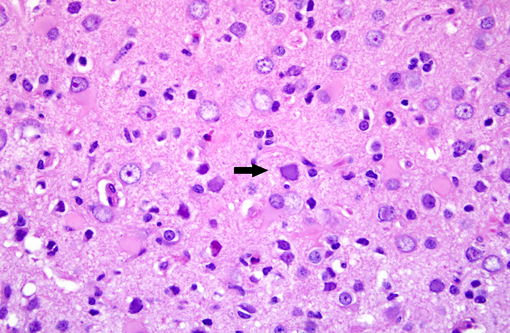
Brain: Multiple zones of malacia and loss of neuropile are present in the cortical grey matter and, in some sections, extend into vacuolated white matter with increased numbers of glial cells. Malacic areas are infiltrated with plump granulated macrophages and smaller numbers of polymorphs. Gemistocytic astrocytes and oligodendrocyte cell bodies are swollen and hyalinized with marginated chromatin and poorly defined to distinct grainy amphophilic intranuclear inclusions. Scattered blood vessels in the surrounding parenchyma have modest cuffs of lymphoid cells or polymorphonuclear leukocytes in malacic areas. Meninges contain mild perivascular lymphoid infiltrates.
Morphologic Diagnosis:
Brain: Multifocal severe subacute meningoencephalitis with multifocal malacia, demyelination and gliosis, SV40
Lab Results:
Multiple clinical nasal swabs contained beta hemolytic coagulase positive staphylococcus. Bacterial culture of the brain was negative.
In situ hybridization of the kidney and brain demonstrates the presence of SV40 entire genomic nucleic acid (nick translation biotinylated probe, Enzo Life Sciences) in nuclei of renal tubule epithelium and oligodendrocytes.
Terminal CBC: 2.7 RBC, 5.5 Hgb, 19.8 Hct, 3.74 WBC, 65 Seg, 1 Eo, 3 Mno. 31 Lym, 150000 Plt, 6.4 Rtc
18 months prior CBC: 5.9 RBC, 14.3 Hgb, 43 Hct,3.8 WBC, 58 Seg, 6 Eo, 30.7 Lym
290000 Plt, 0 Rtc
Terminal Chem: 147 Na, 3.4 K, 111 Cl, 5.8 Pro, 2.1 Alb, 3.7 Glob, 0.6 A/G, 30.6 BUN, 85 Glu, 0.56 Crt
Condition:
Progressive multifocal leukoencephalopathy
Contributor Comment:
SV40 is an oncogenic DNA virus in the Papova virus family and the polyoma virus subfamily. SV40 produces large and small DNA-binding proteins (T antigens) which serve as DNA molecular chaperones with roles in viral replication (transcription and viron assembly) and tumorigenesis(7). The rhesus monkey is the natural host and SV40 persists as a clinically silent renal infection unless immunodeficiency due to Simian Immunodeficiency Virus (SIV) infection allows polyomal viral disease in the kidney, lung or brain. Human polyoma viruses, John Cunningham virus (JVC) and BKV are comparable and subclinical renal infections can be associated with pregnancy, diabetes, or old age. In immunodeficiency, JVC infects oligodendrocytes and causes progressive multifocal leukoencephalopathy (PML) while BKV-infected individuals develop fatal tubulointerstitial nephritis(3). SIV-infected monkeys have two morphologic presentations of polyoma viral disease in the brain; 1) PML characterized by multifocal demyelination with inflammation and gitter cell accumumation(3) and 2) meningoencephalitis (ME) affecting grey matter without significant demyelination(5). Both manifestations display gemistocytic astrocytes and swollen oligodendrocytes with intranuclear inclusions, microgliosis, and T lymphocyte infiltration. It has been suggested that, in monkeys, PML is associated with reactivation of a latent SV40 infection while ME is a manifestation of a primary SV40 infection in younger animals(5). This case has the morphologic lesions of ME and the renal involvement consistent with a primary SV40 infection but the older age of the monkey, the long time duration between SIV inoculation and disease, and presence of demyelination previously linked with PML.
JPC Diagnosis:
Brain: Polioencephalitis, necrotizing, multifocal, severe, with gemistocytes, axonal degeneration, and amphophilic intranuclear viral inclusions.
Conference Comment:
Simian polyomavirus has historical significance, as it was first identified in 1960 in rhesus macaque renal cell cultures used to manufacture both Sabin and Salk polio vaccine, to which millions of inoculants were exposed. The virus is named for its propensity to produce vacuoles in infected cells, hence the name simian vacuolating virus 402. SV40 suppresses p53 expression through the SV40 Large T-antigen and SV40 Small T-antigen, resulting in loss of transcription initiation(7). SV40 can also play a role in development of tumors, most commonly sarcomas, and has been used in rats to develop a model for primitive neuroectodermal tumors (PNETs) and medulloblastomas(1). Additionally, there is ongoing research into the potential of SV40 to cause cancer in humans, as SV40 has been detected in a variety of human cancers, although this topic remains controversial3.
Initially, MHC-I molecules cluster in caveolae and bind to SV40. SV40 then enters the host cell via endocytosis through caveolae, small uncoated invaginations in the host cell plasma membrane important for signal transduction and calcium regulation. Caveolins, membrane bound proteins important to the structure and function of caveolae, associate with the virus membrane, facilitate budding of the virus-containing vesicle into the cell cytoplasm, and the subsequent transfer of virus to the endoplasmic reticulum(6).
There is some variation among slides and, depending on the section, lesion distribution is either focal or multifocal
References:
1. Horvath CJ, Simon MA, Bergsagel DJ, Pauley DR, King NW, Garcea RL, Ringler DJ: Simian Virus 40-induced disease in rhesus monkeys with simian acquired immunodeficiency syndrome.Â
Am J Path 140(6): 1431-1440, 1992
2. Simon MA, Ilyinskii PO, Baskin GB, Knight HY, Pauley DR, Lackner AA: Association of Simian Virus 40 with a central nervous system lesion distinct from progressive multifocal leukoencephalopathy in macaques with AIDS.Â
Am J Path 145(2): 437-446, 1999
3. Sullivan CS and Pipas JM: T antigens of Simian Virus 40: Molecular Chaperones for viral replication and tumorigenesis.Â
Microbiol Molec Biol Rev 66(2):179-202, 2002
4. Fiers W et al., Complete nucleotide-sequence of SV40 DNA, Nature, 273, 113-120, 1978
5. Eibl RH, Kleihues P, Jat PS, Wiestler OD (1994) A model for primitive neuroectodermal tumors in transgenic neural transplants harboring the SV40 large T antigen.Â
Am J Pathol. 1994 Mar;144(3):556-64
6. Rollison DEM, Page WF, et al. Case-Control Study of Cancer among US Army Veterans Exposed to Simian Virus 40-contaminated Adenovirus Vaccine.Â
American Journal of Epidemiology. 2004 Aug;160(4):317-24.
7. Stang E, Kartenbeck J, Parton RG. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae.Â
Mol Biol Cell. 1997 Jan; 8(1):47-57.